Question
Question: Which product is formed when 3-methylpentane-1,3,4-triol is heated under reflux with an excess of ac...
Which product is formed when 3-methylpentane-1,3,4-triol is heated under reflux with an excess of acidified potassium dichromate (VI)?
A.HO2CCH2C(CH3)(OH)COCH3
B.HO2CCH2COC(OH)(CH3)2
C.OHCCH2C(CH3)(OH)COCH3
D.HO2CCH2CO(CH3)COCH3
Solution
Primary alcohols can be oxidized to either aldehydes or carboxylic acids depending on the reaction conditions. In the case of the formation of carboxylic acids, the alcohol is first oxidized to an aldehyde which is then oxidized further to the acid. Secondary alcohols are oxidized to ketones - and that's it. For example, if we heat the secondary alcohol propan-2-ol with sodium or potassium dichromate (VI) solution acidified with dilute sulphuric acid, you get propanone formed. Tertiary alcohols are not oxidized by acidified sodium or potassium dichromate (VI) solution - there is no reaction whatsoever.
Complete step by step answer:
Primary alcohols can be completely oxidized (or aldehydes can be further oxidized) to form carboxylic acids on stronger heating and refluxing with excess acidified dichromate (VI).
Secondary alcohols are oxidized to form ketones.
Tertiary alcohols are not oxidized by acidified dichromate (VI) ions. The dichromate (VI) ions remain orange.
When heated with acidified aqueous potassium dichromate (VI), alcohols can be oxidised (dichromate ions are powerful oxidising agents) to form a compound with a carbonyl group. The orange dichromate ions are reduced to green chromium (III) ions in this reaction.
The acidified potassium dichromate (VI) will oxidise −OH group attached primary carbon to carboxylic group and secondary carbon to ketone.
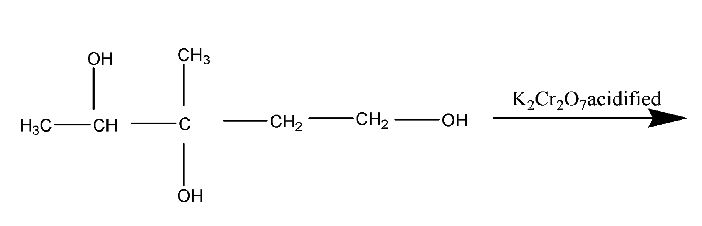
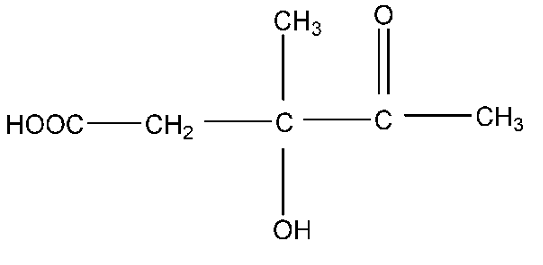
Therefore, the correct answer is option (A).
Note: Tertiary alcohols are not oxidized by acidified dichromate (VI) ions. The oxidizing agent is removing the hydrogen from the −OH group, and a hydrogen from the carbon atom attached to the −OH. Tertiary alcohols don't have a hydrogen atom attached to that carbon.
