Question
Question: Which plot is the adsorption isobar for chemisorption where \[x\] is the amount of gas adsorbed on m...
Which plot is the adsorption isobar for chemisorption where x is the amount of gas adsorbed on mass m(at constant pressure) at temperature T?
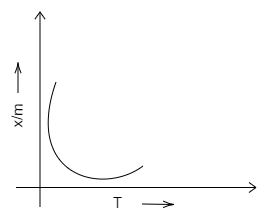
A.
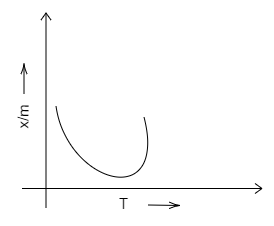
B.
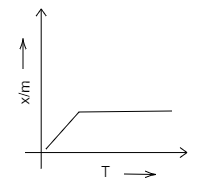
C.
D.
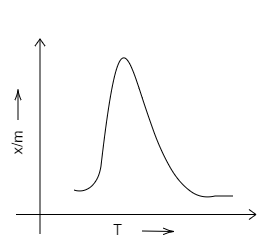
Solution
Chemisorption is used for the evaluation of the number of active sites available to increase the rate of, or the catalyse, chemical reactions. Adsorption isobar is a graph plotted between the amount of the adsorbed (x/m) and that of the temperature (T) of the adsorbate at a constant pressure.
Complete step by step answer:
We know that chemical adsorption initially increases, and then it decreases to attain constancy with that of the rise in temperature. It is because of the heat supplied that acts as activation energy. We can draw the graph in the following way.
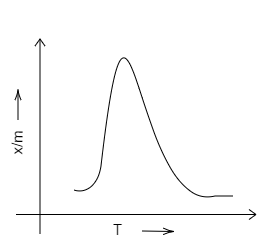
Therefore, we can conclude that D is the correct option. A, B and C are incorrect options.
Additional information:
We know that there are some features that help us in recognizing chemisorption. These include:
(a) Chemisorption is characterized by specificity of chemical.
(b)In chemisorption, we can detect the changes in the electronic state by some physical means such as e.g. U.V, magnetic susceptibility, microwave or infrared spectroscopy and electrical conductivity etc.
(c) We can alter the chemical nature of the adsorptive by surface dissociation or by the reaction in such that on desorption, the original species is not recoverable. Hence, chemisorption might not be reversible in that case.
(d) The order of magnitude of energy of the chemisorption is the same as the change in energy in a chemical reaction between a fluid and solid and a fluid. So, a chemical reaction can be either exothermic or endothermic.
(e) An activation energy is involved in the elementary step in chemisorption.
Note:
Chemisorption measurement techniques are useful for evaluating the physical and the chemical properties of the materials that are critical for the reaction performance. Chemisorption measurements are important for the characterization of the catalysts which are used in industries such as oil and gas, petrochemicals and fine chemicals, environmental and many others etc.
