Question
Question: Which oxyacid of sulphur contains S-S single bond? A.Oleum B.Marshall’s acid C.Dithionic acid ...
Which oxyacid of sulphur contains S-S single bond?
A.Oleum
B.Marshall’s acid
C.Dithionic acid
D.Thiosulphuric acid
Solution
In oxyacid of sulphur, sulphur is the central atom exhibiting a tetrahedral structure when coordinated with oxygen. This contains at least one S=O bond and one S−OH bond. In addition to these, a chain of (−S−)n as in H2S2O6 . Such oxyacids with S−S linkages are called Thioacids.
Complete step-by-step solution:
The sulphur oxyacids are the acids that contain oxygen, hydrogen and sulphur. These are divides into four groups based on their structural similarities:
Sulphurous acid group- these are prepared by dissolving sulphur dioxide in water. Their structure is pyramidal with three oxygen atoms on a triangle i.e. tetrahedral structure is distorted by lone pairs. These are strong reducing agents and have bleaching properties.
For example, sulphurous acid H2SO3 .
Sulphuric acid group- these are also called oil of vitriol, produced by lead chamber and contact processes by dissolving SO3 in water. For example, sulphuric acid H2SO4 (oleum) and thiosulphuric acid.
Thionic acid group- these are a series of unstable acids with general formula of H2SnO6 where n=2 to 6. For example, Dithionic acid H2S2O6 .
Peroxo acid group- these are also called Marshall’s acid or Caro’s acid. The central atom sulphur has +6 oxidation state with a peroxo group in it. These are derived from hydrogen peroxide by replacing hydrogen atoms. For example, peroxydisulfuric acid H2S2O8 .
From all these series, we saw that the S−S bond is present only in thionic acid series. For example, dithionic acid H2S2O6 .
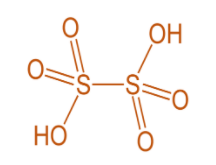
Hence, the correct option is (C).
Note: All oxyacids have the acidic hydrogen bonded to an oxygen atom. i.e. −OH bond. The electronegativity of the central atom and the number of O atoms are responsible for the oxyacid acidity.
