Question
Question: Which oxyacid of sulphur contains S-S single bond? A. Oleum B. Marshall’s acid C. Dithionic ac...
Which oxyacid of sulphur contains S-S single bond?
A. Oleum
B. Marshall’s acid
C. Dithionic acid
D. Thiosulphuric acid
Solution
Oxyacid is a compound that contains oxygen, hydrogen and one other element. Here, we are provided with oxoacids of sulphur, these contain oxygen, hydrogen and sulphur atoms.
Complete step by step answer:
- Oleum is also called as pyrosulfuric acid. The molecular formula of oleum is H2S2O7. We can see the structure of oleum:
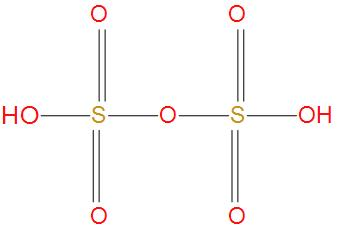
- We can see from the structure that there is no S-S single bond present, hence this option is wrong.
- Next option is Marshall’s acid, we also called it as peroxydisulfuric acid, its molecular formula is H2S2O8 . We will see the structure of this:
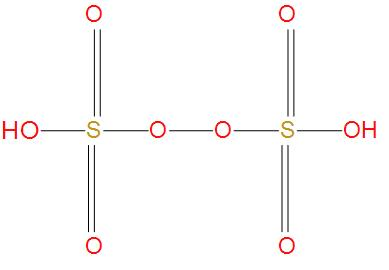
- Here, we can see that there is one peroxy linkage that O-O is present, and there is no S-S single bond present. Hence, this option is wrong.
- Let’s see the next option Dithionic acid, its molecular formula is H2S2O6. We will see the structure of this:
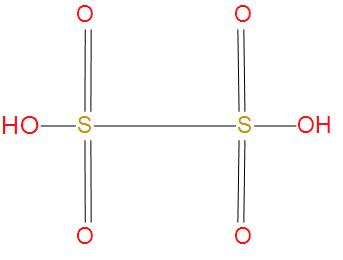
- We can see from the structure that there is one S-S single bond present. And we can say that this option is correct.
- Similarly, in Thiosulphuric acid, there is no S-S single bond present. We can see this from structure:
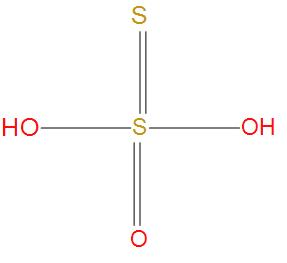
Hence, we can conclude that the correct option is (C), Dithionic acid contains S-S single bond.
Note: We know that an oxyacid molecule contains the structure X-O-H, where O is oxygen, H is hydrogen and X is any element. Where, other atoms can be connected to the central metal atom X. In a solution, oxyacid can be dissociated as: X−O−H⇄(X−O)−+H
