Question
Question: Which one of the following will be reactive for Perkin Condensation? A. 
B.
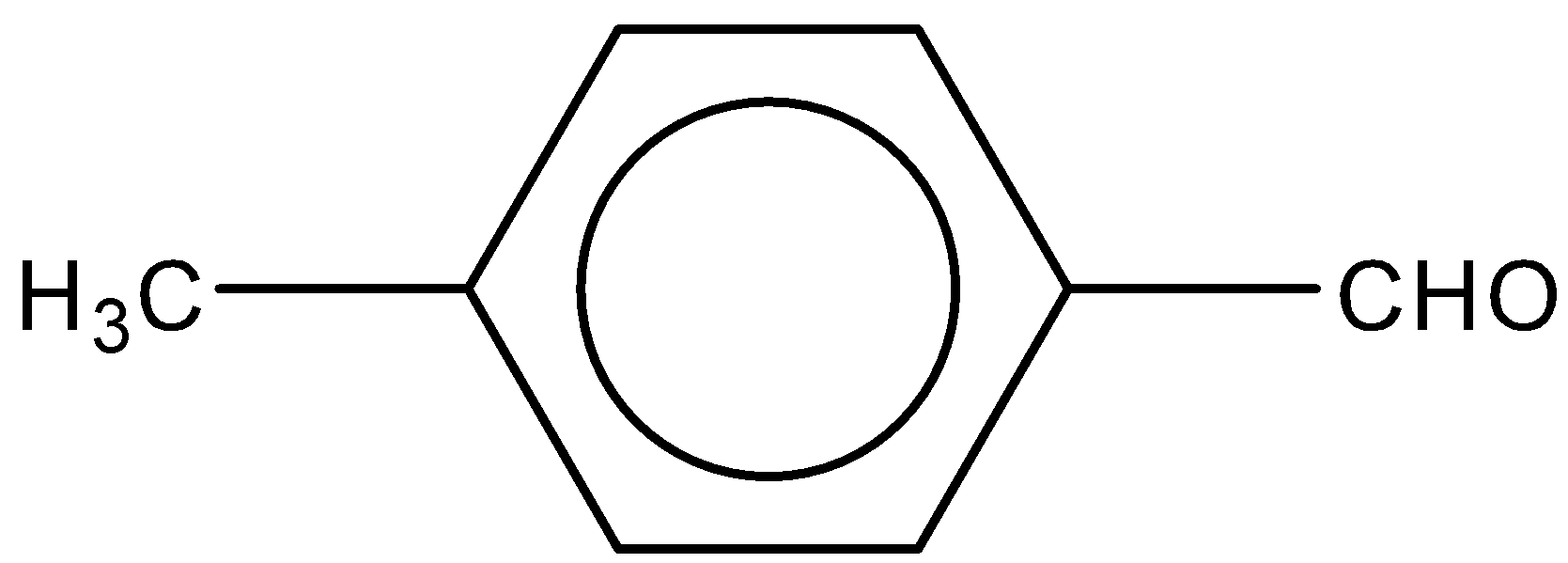
C.
D.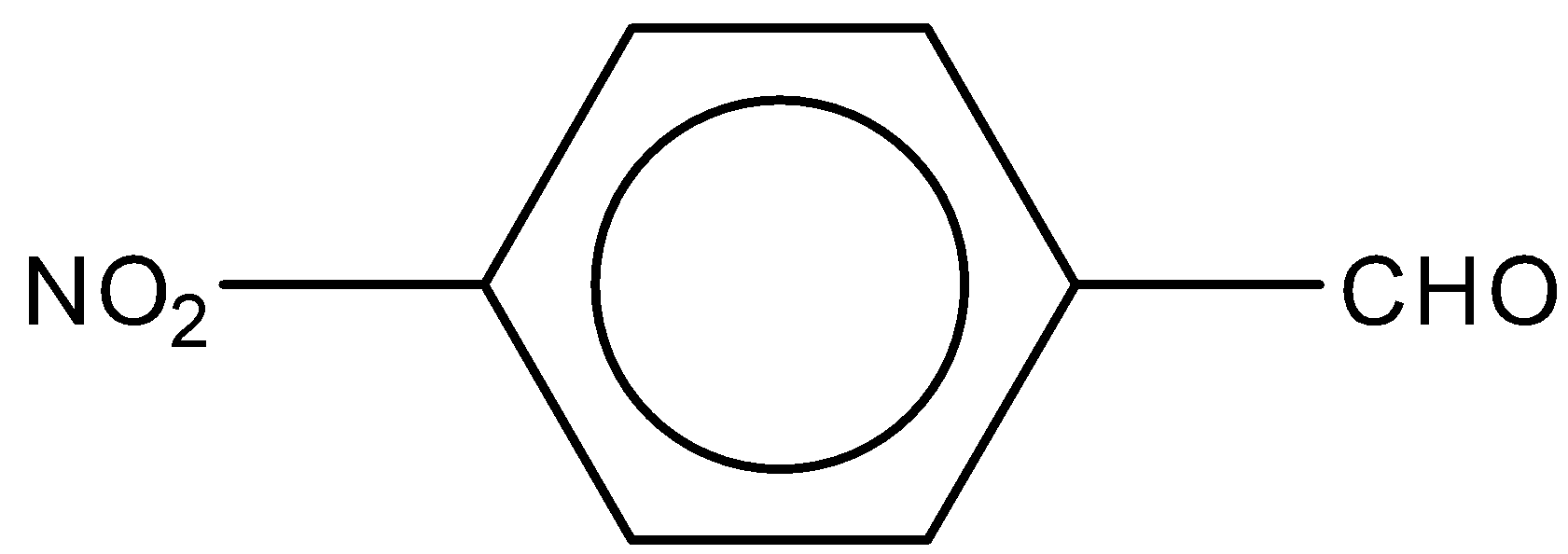
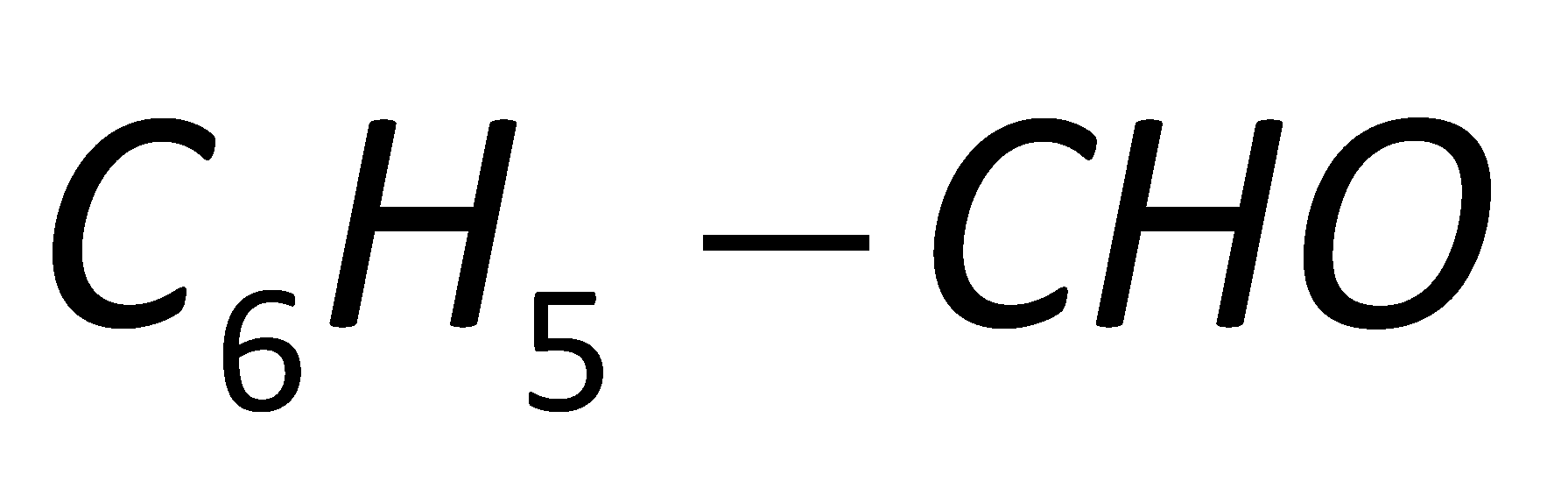
Solution
Perkin condensation involves the reaction of aromatic aldehyde, aliphatic acid anhydride, and alkali salt and the product attained are the derivatives of cinnamic acid. The reactivity towards Perkin condensation depends upon the electron donating or electron withdrawing group attached to the benzene ring.
Complete step by step answer:
Now, let us discuss Perkin condensation in detail.
As mentioned, the reactants involved are aromatic aldehyde, aliphatic acid anhydride, and an alkali salt. The general reaction can be represented as follows:
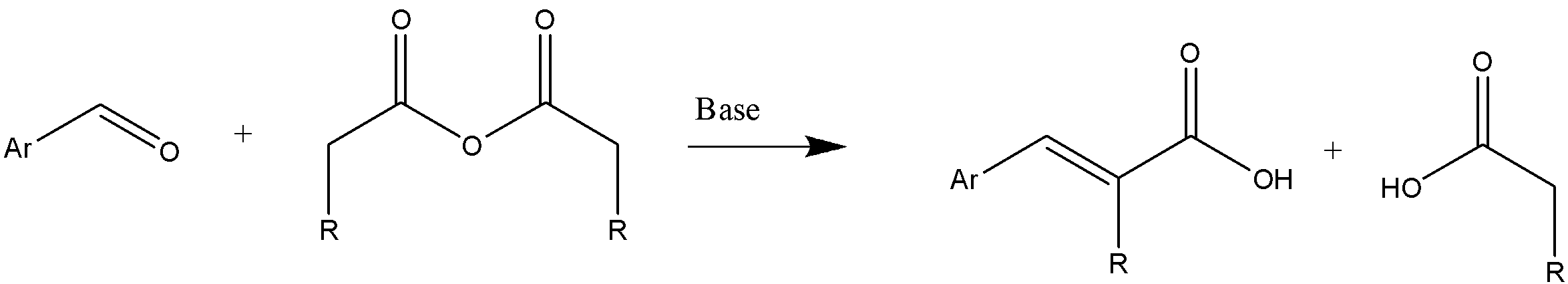
Here, in the general reaction we can see that alkali salt behaves as a base catalyst. The necessary condition for Perkin condensation is the presence of minimum 2 α hydrogen in a molecule.
As we know that the reactivity towards Perkin condensation depends upon the nature of the group attached to it. If the electron withdrawing group is attached to the ring it will lead to increase in reactivity and the electron releasing (donating) groups will lead to the decrease in the reactivity.
Now, if we look at the given options, in the A and B option, OCH3andCH3 groups are attached to the benzene ring. Both the groups are electron donating groups in nature. OCH3 is more electron donating than CH3 as lone pairs are present on the oxygen atom in OCH3. So, we can say that these two will decrease the reactivity towards Perkin condensation.
In the third option C, the nitro group (NO2) is attached to the benzene ring. NO2 group acts as an electron withdrawing group in nature. During the reaction it increases the charge on the carbonyl group. Therefore, we can say it increases the reactivity towards Perkin condensation.
In the fourth option D, no group is attached to the benzaldehyde. Thus, it will not show any effect towards the Perkin condensation.
So, the correct answer is Option C.
Note: In the question we came across a factor that OCH3is more electron donating group than CH3 because CH3 group shows hyperconjugation and +I effect whereas OCH3 shows +M effect. Thus, in aromatic electrophilic substitution reaction OCH3 group will be more effective towards the formation of ortho and para substituents.
