Question
Question: Which one of the following statements is True: 
(A) PhLi adds to both compounds with equal ease
(B) PhLi does not add to either of the compounds
(C) PhLi reacts readily with 1 but does not add to 2
(D) PhLi reacts readily with 2 but does not add to 1
Solution
To solve this question, we must first some basic knowledge about Phenyllithium (PhLi) and also we must understand the basic concepts of Aromaticity. Then we need to assess the properties of PhLi and then use the rules for Aromaticity to determine that what will be the action of PhLi on 1and2 and then only we can conclude the correct answer.
Complete step-by-step answer: PhLi : Phenyllithium or lithobenzene is an organometallic agent with the empirical formula C6H5Li . It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses.Aromaticity: It is defined as a property of the conjugated cycloalkenes which enhances the stability of a molecule due to the delocalization of electrons present in the π-π orbitals.
The aromatics compounds are said to exhibit some special characteristics or called as rules which are given below-
1.Aromatic compounds are always cyclic structures.
2.Each element of the ring within the structure must and should have a p-orbital ring which is in a perpendicular form to the ring, and this makes it a planar molecule
3.All the compounds obey the Huckel’s Rule, i.e. all the aromatic compounds should have the (4n+2)π number of electrons.
4.The organic compound has to be flat.
Step 1: For the first compound:
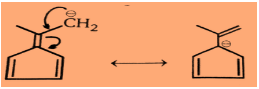
So very clearly we can see in the above structures that it is an aromatic compound.
Step2:For the second compound:
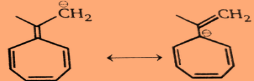
So very clearly we can see in the above structures that it is an antiaromatic compound.And Hence, the first compound will be more reactive towards PhLi and the second compound will be unreactive towards PhLi .Conclusion: PhLi reacts readily with 1 but does not add to 2
So, clearly we can conclude that the correct answer is Option (C).
Note: Crystalline phenyllithium is colorless; however, solutions of phenyllithium are various shades of brown or red depending on the solvent used and the impurities present in the solute.
