Question
Question: Which one of the following species does not exist under normal conditions? A.\(B{e_2}^ + \) B.\(...
Which one of the following species does not exist under normal conditions?
A.Be2+
B.Be2
C.B2
D.Li2
Solution
This question could be approached using MOT which stands for molecular orbital theory in which the number of electrons in BMO (Bonding Molecular Orbital) and in ABMO (Antibonding Molecular Orbital) can be used to calculate the bond order of the molecule, which decides the stability of the molecule.
Complete Step by step solution:
The bond order is the number of bond of bond which is present in between two atoms, which can be calculated using number of electrons in BMO and ABMO by the formula given below:
Bond order = 21(NB−NA), where NB stands for the number of electrons in the BMO and NA stands for the number of electrons in the ABMO. Now we can draw the MO diagram of Be2 and find outs bond order.
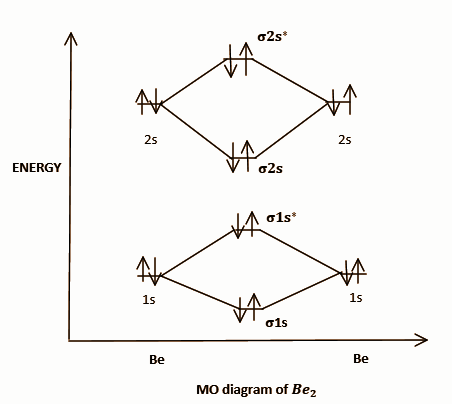
Be electronic configuration is:1s22s2.
This diagram can be represented by equation given as follows:
σ1s2σ∗1s2σ2s2σ∗2s2 , where σ stands for the sigma bonds between atoms which are single bonds and ∗ represents the antibonding orbitals. Now, we have a total of four electrons in bonding and 4 electrons in antibonding orbitals. So using the formula:
Bond order= 21(NB−NA)= 21(4−4)=0
Since bond order is 0 it means there is no bond between two Be atoms and hence the Be2 molecule will be unstable. For other options we can similarly draw the molecular orbital diagrams and can find out the bond order.
For Be2+ one electron will be removed from the antibonding orbital and hence NB=4 and NA=3. So the bond order =21(NB−NA)=21(4−3)=21. This means there is a partial bond between them and hence stable.
For Li the electronic configuration is 1s22s1 and the Li2 molecular orbital can be written by the equation σ1s2σ∗1s2σ2s2 and the bond order will be 21(NB−NA)=21(4−2)=1 which means there is a single bond between them, so stable.
For B the electronic configuration is 1s22s22p1 and the B2 molecular orbital will be written by the equation σ1s2σ∗1s2σ2s2σ∗2s2π2px1π2py1 . So, NB=6 and NA=4 and bond order is 21(6−4)=1 and hence there is single bond between two boron atoms and hence boron molecule will be stable.
Note: In case of boron atom it involves p orbitals and all the three p orbitals px,py,pz are not equivalent while electrons are filled, px,py have lower energy than pz where total electrons of the molecule are 14 or less than 14.
