Question
Question: Which one of the following properties is not shown by NO? (A) It combines with oxygen to form nitr...
Which one of the following properties is not shown by NO?
(A) It combines with oxygen to form nitrogen dioxide.
(B) Its bond order is 2.5.
(C) It is diamagnetic in gaseous state.
(D) It is a neutral oxide.
Solution
Think about the properties of nitric oxide. Write the electronic configuration of nitrogen and oxygen and then try to draw the molecular orbital diagram of nitric oxide, NO. Then calculate the bond order of NO and check its magnetism. Write the reaction of NO with oxygen and see if NO dissolves in water and its reactivity with acids and bases and then select the correct option.
Complete step by step answer:
- NO is nitric oxide. NO reacts with oxygen to form nitrogen dioxide. This reaction is used to prepare nitrogen dioxide and is given as, 2NO+O2→2NO2
- Nitrogen has the atomic number 7 and oxygen has the atomic number 8. Their electronic configurations are given as,
7N=1s22s22p3
8O=1s22s22p4
- Molecular orbital diagram of nitric oxide can be represented as,
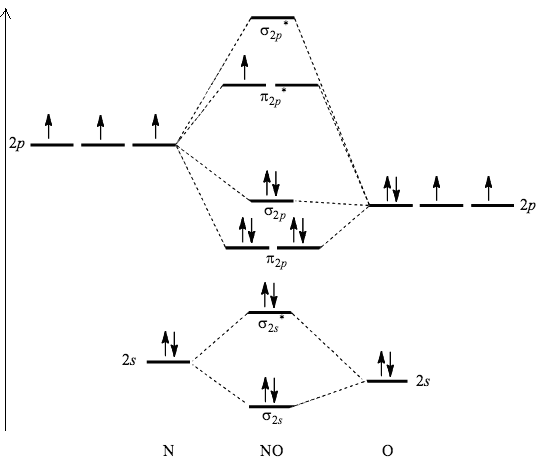
- Now, we can see that an anti-bonding orbital of NO has one unpaired electron and so, it is paramagnetic in nature.
- The bond order of NO is calculated as 28−3=25=2.5
- Nitric oxide dissolves in water and doesn’t dissolve in acids and bases. Therefore, NO is a neutral oxide.
So, the correct answer is “Option D”.
Note: Remember nitric oxide is a neutral oxide and it reacts with oxygen to form nitrogen dioxide. Nitric oxide is paramagnetic in nature due to the presence of an unpaired electron. Nitric oxide will be resonance stabilized and hence have a partial triple bond character and therefore, its bond order 2.5 is correct.
