Question
Question: Which one of the following is the strongest acid? A.
B. 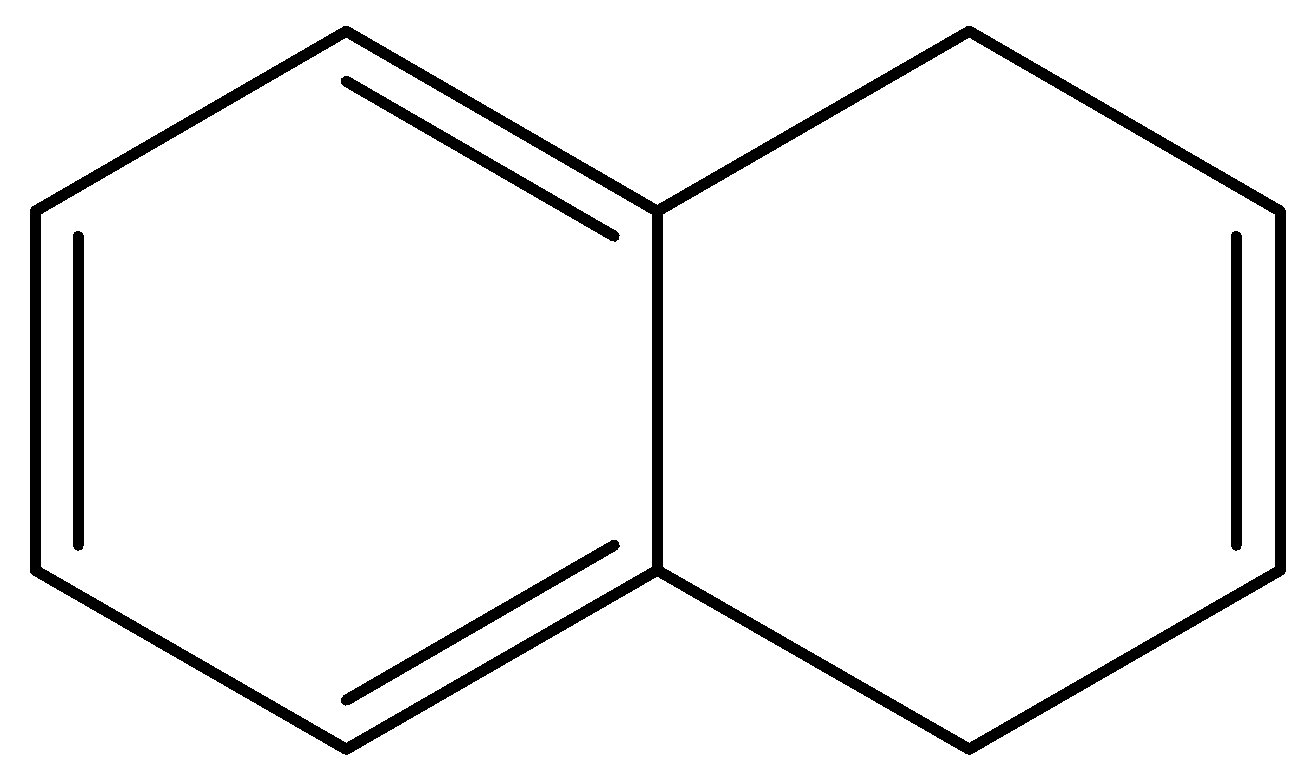
C. 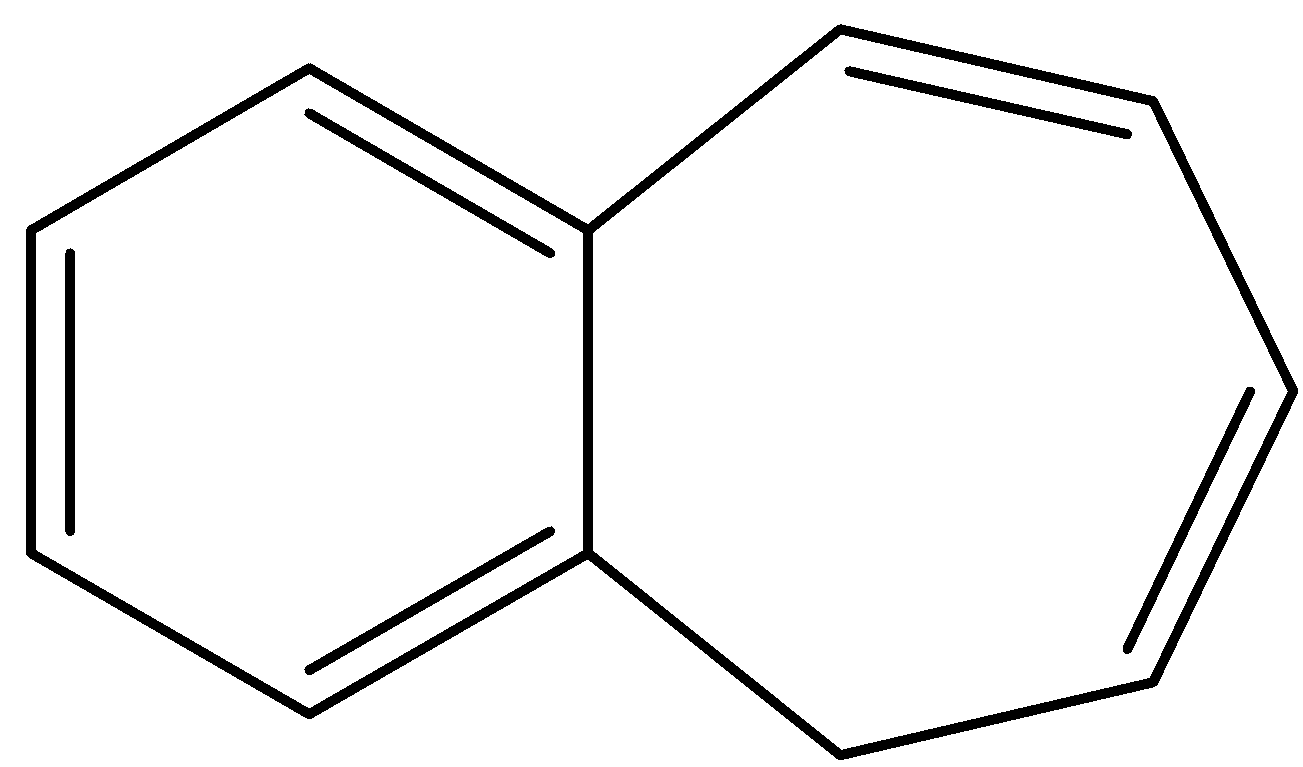
D. None of the above
Solution
If a compound is going to donate hydrogen ion easily then the compound is called an acid and after donating the compound will get an opposite charge to the compound nothing but a negative charge on the counterpart.
Complete answer:
- In the question it is asked to find the strongest acid among the given options.
- Coming to the given options, option A,
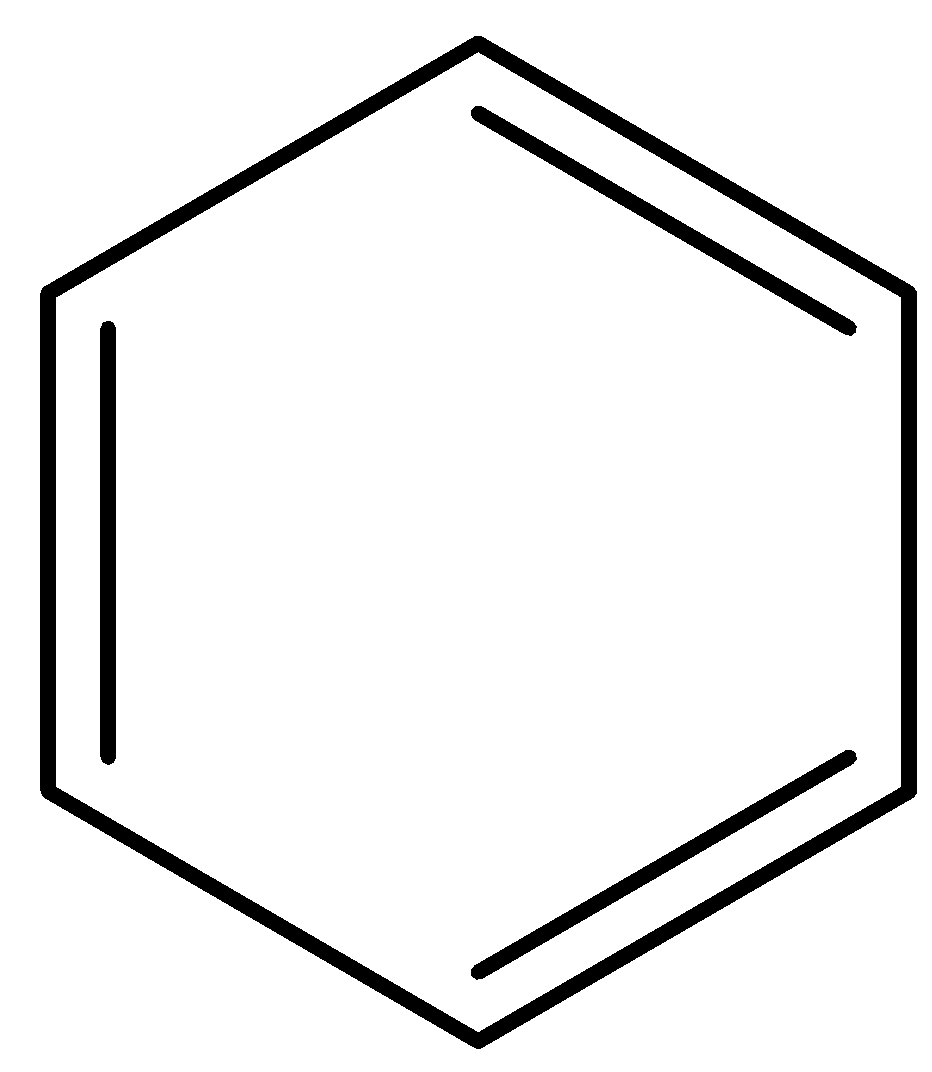
- The compound in the option A is benzene.
- We know that benzene is more stable in nature and not going to donate any hydrogen ion to others.
- Therefore the option A is incorrect.
- Coming to option B,
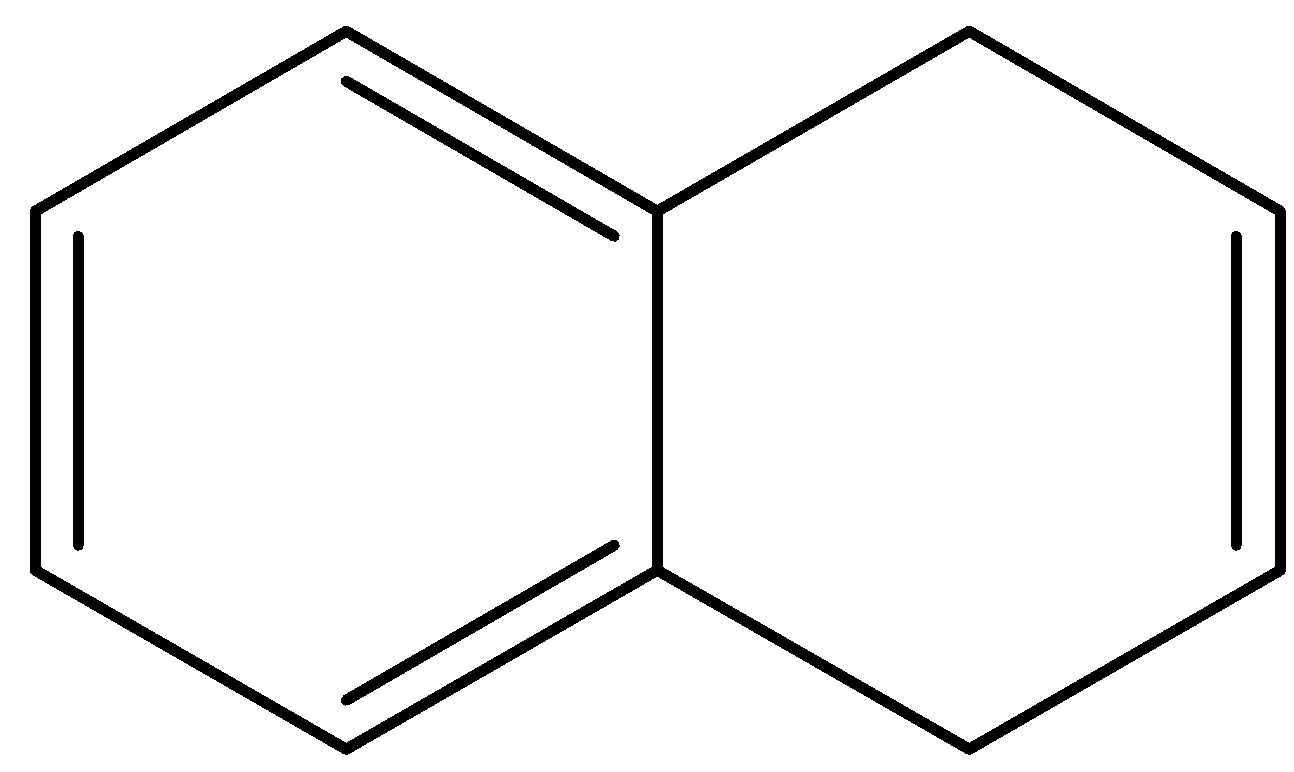
- The compound B is dihydro naphthalene. It will donate a hydrogen ion and shows the following resonance structures.
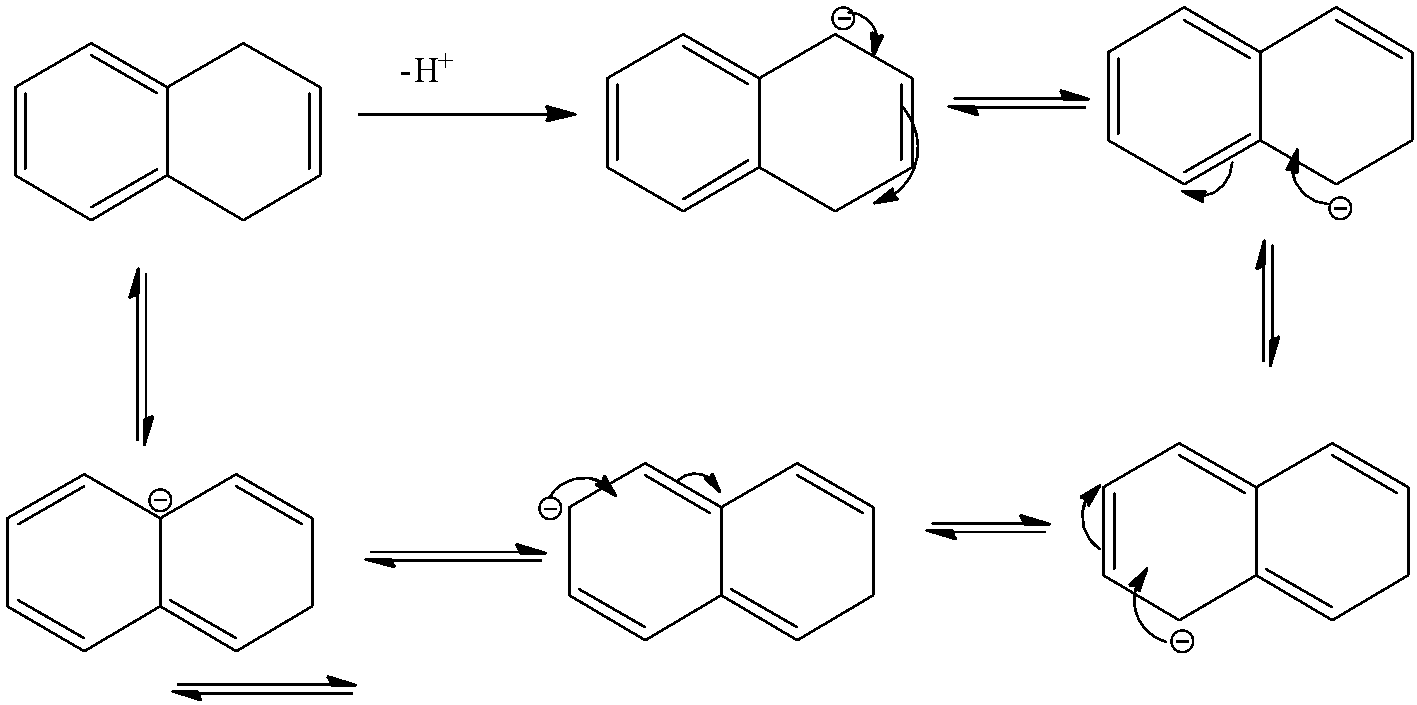
- Therefore the compound B is acidic in nature because after donating a hydrogen ion there is a generation of number of resonance structures and it makes the molecule B is more stable.
- Coming to option C,
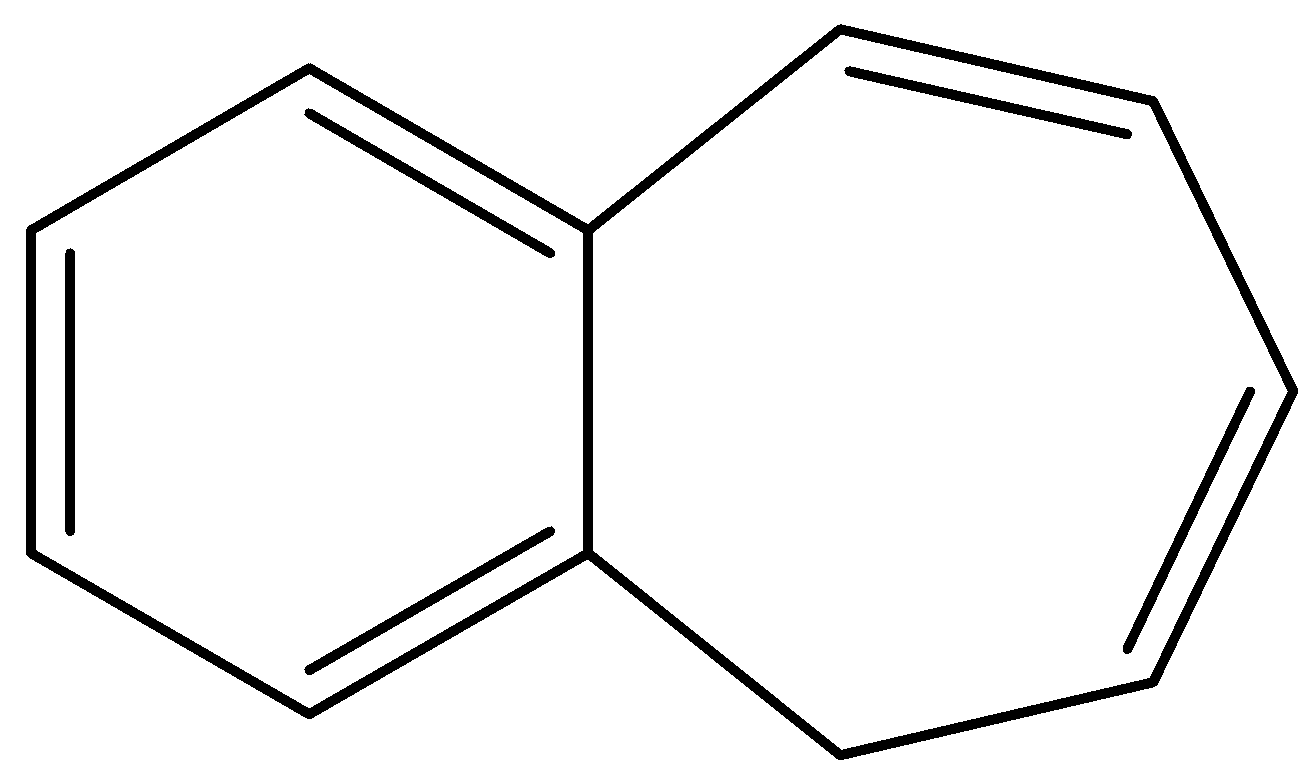
- Compound C also donates a hydrogen ion and shows the following resonance structures.
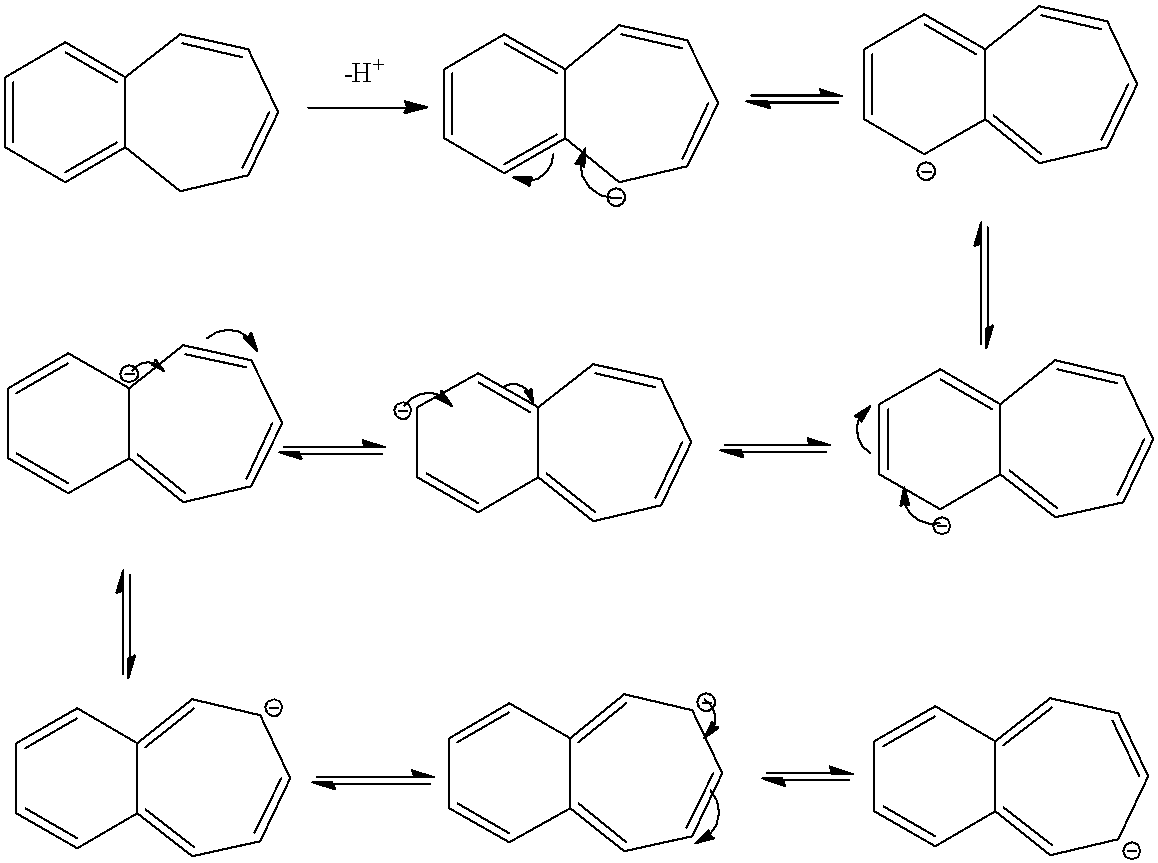
- Therefore the compound C is acidic in nature because after donating a hydrogen ion there is a generation of number of resonance structures and it makes the molecule c is more stable.
- When compared to compound B, compound C is more acidic due to the formation of more resonance structures by the compound C when compared to compound B.
- Therefore the strongest acid among the given options is C.
So, the correct option is C.
Note:
The acidity of the organic compounds directly depends on the number of resonance structures going to be formed by the respective organic compounds. After losing a hydrogen ion we organic compounds show the acidic nature.
