Question
Question: Which one of the following is the correct electronic configuration of sodium? A.\(2,8\) B.\(8,2...
Which one of the following is the correct electronic configuration of sodium?
A.2,8
B.8,2,1
C.2,1,8
D.2,8,1
Solution
Electronic configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. It also describes how each electron moves independently in an orbital and also allows us to understand the shape and energy of its electrons. There are many general rules that are taken into consideration while assigning the location of the specific electron to its energy state. Knowing the electronic configuration of a species gives us a better understanding of its bonding ability, magnetism and other chemical properties.
Complete answer: The arrangement and distribution of electrons in different orbit is based on the Bohr-Bury model. The maximum number of electrons that can be present in a particular shell can be determined by using the formula - 2n2, where n is the number of energy shells.
There are four different shells as given by Bohr, namely K,L,M and N and the order of these energy shells goes likeK<L<M<N.
So, the maximum number of electrons that can be present in these shells can be determined by using the formula 2n2.
For K shell, n=1
Therefore maximum number of e− =2n2=2×1=2
For Lshell, n=2
Therefore maximum number of e−=2n2=2×(2)2=8
For Mshell, n=3
Therefore maximum number of e−=2×(3)2=18
For N shell, n=4
Therefore the maximum number of e−=2×(4)2=32.
In the question, we are given sodium as the element. We know, the atomic number of sodium is 11. Therefore, we can apply the Bohr-Bury rule to find out the electron distribution as well as the electronic configuration. So, according to the Bohr-Bury rule, electrons can be distributed as 2,8,1.
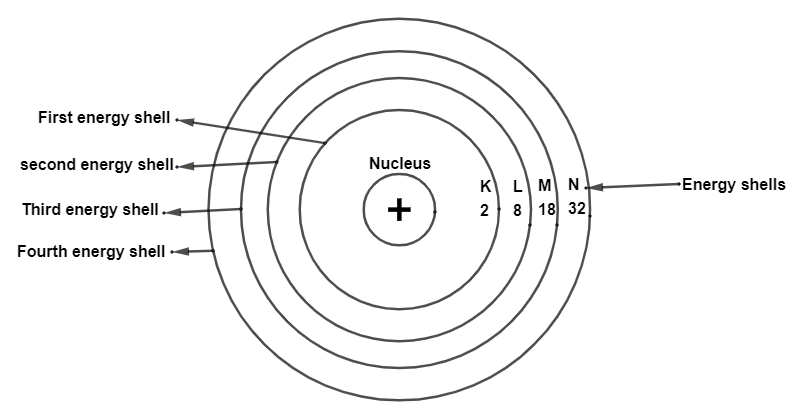
Fig: a few energy levels in an atom.
Therefore, option (d) 2,8,1 is the correct electronic configuration of sodium.
Note:
Although the outermost shell has the capacity to take upto 32 electrons, it can only take 8 electrons. This is called octet rule and the electron can only go to the outer shell when the inner shells are completely filled.
Chemically active elements do not have octet electrons in their valence shell. Their activity rises from the tendency to achieve the octet by forming bonds.
