Question
Question: Which one of the following is not an allylic halide? (A) 4-Bromopent-2-ene. (B) 3-Bromo-2-methyl...
Which one of the following is not an allylic halide?
(A) 4-Bromopent-2-ene.
(B) 3-Bromo-2-methylbut-1-ene.
(C) 1-Bromobut-2-ene.
(D) 4-Bromobut-1-ene.
Solution
Allylic halides are compounds in which halogen atoms attached to sp3 hybridized carbon atom next to carbon-carbon double bond (C = C).
The skeleton of an allylic is C=C-C.
Example: CH3−CH=CHCH2Cl is an allylic halide.
Complete step by step answer:
Let us discuss given options one by one by drawing structures.
(1) 4-Bromopent-2-ene
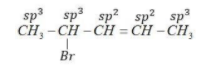
Bromine atom is attached to sp3 C-atom and next to sp2 hybridized carbon atom.
This is an allylic halide.
(2) 3-Bromo-2-methylbut-1-ene
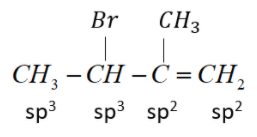
Bromine atom attached to sp3 hybridized C-atom next to sp2 hybridized C-atom.
This is an allylic halide.
(3) 1-Bromobut-2-ene
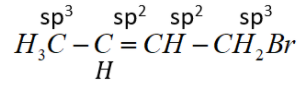
Bromine attached to sp3 hybridized C-atom.
This is an allylic halide.
(4) 4-Bromobut-1-ene
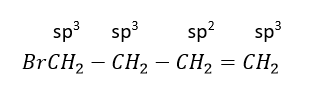
Bromine is attached to sp3 C-atom next to sp3 hybridized C-atom.
Therefore, this is not an allylic halide.
Therefore, from the above explanation the correct option is (D) 4-Bromobut-1-ene is not an allylic halide.
Note: Alkyl halide is less stable than vinyl halide because C-X bond in vinyl halide is stronger due to more double bond character.
While identifying allylic halide, write a type of hybridization on each C-atom and find to which Br-atom is attached.
