Question
Question: Which one of the following is not a Lewis acid? A. \(B{F_3}\) B. \(AlC{l_3}\) C. \(BeC{l_2}\...
Which one of the following is not a Lewis acid?
A. BF3
B. AlCl3
C. BeCl2
D. SnCl2
Solution
We can use the definition of Lewis acid to deduce which one of the given compounds cannot be classified as a Lewis acid. The electron deficient compounds are considered as Lewis acids.
Complete answer
We know that various efforts were made to explain the properties of matter with different theories for bonding between the atoms. One such theory was given by Lewis. He proposed that atoms combine to complete their octet and a bond is formed. We can take the example of formation of N2. Here, we have two atoms, both of nitrogen. We can write the Lewis symbols for these by representing the valence electrons as dots:

As we can see that both the atoms have 5 valence electrons and need 3 more to complete their octet. In order to do that they share 3 pairs of electrons that can be shown as follows:
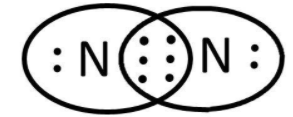
We know that this sharing of valence electrons result in covalent bond formation. So, the bonding can be shown as:
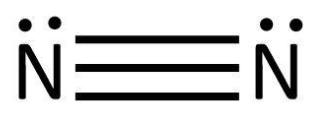
Lewis took it further and defined acids and bases as molecules which accept and donate electrons respectively while forming a bond. Based on this, we will examine the Lewis structures of the given molecules and try to deduce whether they are Lewis acids or not.
BF3: here, we have 3 and 7 valence electrons from B and F respectively. Let’s write its Lewis structure:
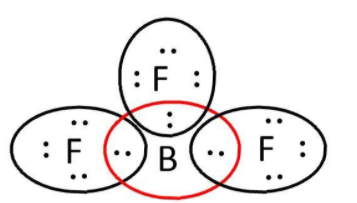
As we can see that octet of all 3F are complete but not that of boron. So, it is electron deficient and can act as a Lewis acid.
AlCl3: here, we have 3 and 7 valence electrons from Al and Cl respectively. Let’s write its Lewis structure:
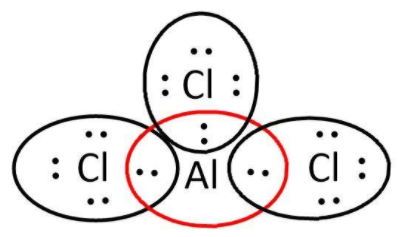
As we can see that octets of all 3Cl are complete but not that of aluminum. So, it is electron deficient and can act as a Lewis acid.
BeCl2: here, we have 2 and 7 valence electrons from Be and Cl respectively. Let’s write its Lewis structure:
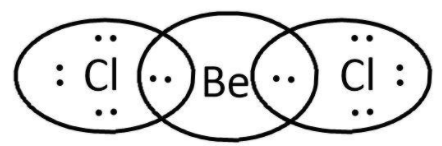
As we can see that octets of both Cl are complete and Be cannot accommodate more electrons as well for its valence orbital is filled. So, it is not electron deficient and cannot act as a Lewis acid.
SnCl2: here, we have 4 and 7 valence electrons from Sn and Cl respectively. Let’s write its Lewis structure:
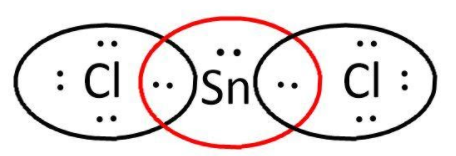
As we can see that octets of both Cl are complete but not that of Sn and it can also use its d-orbitals. So, it is electron deficient and can act as a Lewis acid.
**Hence, the correct option is C.
Note: **
There are some molecules that do not follow the octet rule such as BeCl2 so we can’t rely solely on octet rule for electron deficiency.
