Question
Question: Which one of the following is more acidic? A. 
B. 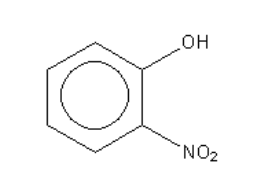
C. 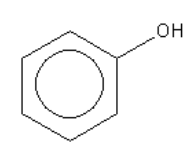
D. 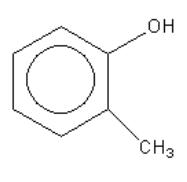
Solution
The strength of an acid depends on the ability of acid to donate the proton and stability of the conjugate base. Electron donating group decreases the acidity of acid and electron-withdrawing group increases the acidity of acid.
Complete step by step answer:
The strength of an acid depends on the ability of acid to donate the proton. Acids having a greater ability to donate the protons are known as a strong acid.
Out of the given acids compound given in option A is alcohol. Alcohols are weaker acid than the phenol as the conjugate base of phenol is stabilized by resonance structure.
Resonance structures of phenol are as follows:
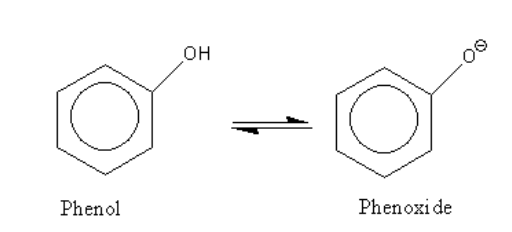
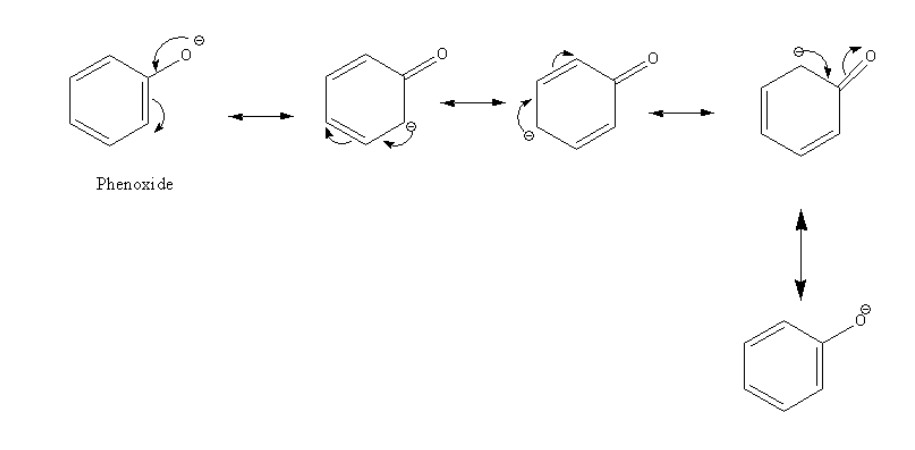
The acidic strength of substituted phenol is dependent on the nature of the substituent.
The electron-withdrawing group decreases the electron density of the phenol ring and stabilizes the negative charge and thus increases the acid strength.
In the case of the compound given in option B (ortho-nitro phenol) the substituent is a nitro group ( - NO2 ). It is an electron-withdrawing group; it increases the acidity so ortho-nitro phenol is a stronger acid than phenol.
Electron donating substituent increases the electron density of the phenol ring and destabilizes the negative charge and thus decreases the acidity.
In the case of the compound given in option D (ortho-cresol) the substituent is a methyl group ( - CH3 ). It is an electron-donating group, it decreases the acidity so ortho-cresol is a weaker acid than phenol.
So the decreasing order of acid strength of a given compound is as follows:
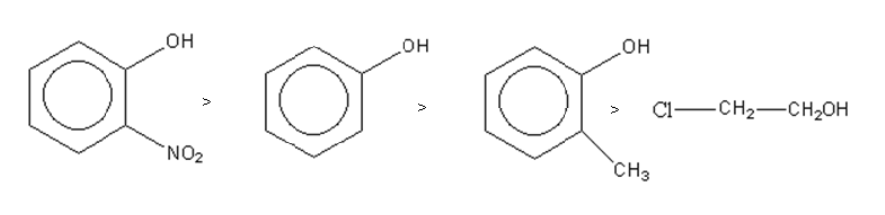
**So, option (B) is the correct answer.
Note:**
Ring activating groups that are electron-donating groups decreases the strength of acid while a ring deactivating group increases the strength of the acid. Alkyls are electron-donating groups. Halides and nitro groups are electron-withdrawing groups.
