Question
Question: Which one of the following is likely to give a precipitate with \(AgN{O_3}\) solution? (A) \({(C{H...
Which one of the following is likely to give a precipitate with AgNO3 solution?
(A) (CH3)3CCl
(B) CHCl3
(C) CH2=CHCl
(D) CCl4
Solution
. If the compound can give free chloride ions in its solution, then it will react with silver nitrate solution to give white precipitates of AgCl (Silver chloride). Amongst all carbocations, the tertiary carbocations are most stable.
Complete step by step answer:
We know that silver nitrate (AgNO3) can give precipitates in a solution which contains chloride ions.
- Silver nitrate reacts with the chloride ions to give AgCl (Silver Chloride) which gets precipitated in the solution because of its very low solubility. So, we will take a look at all the given compounds to find out which one will have free chloride ions in its solution.
A) 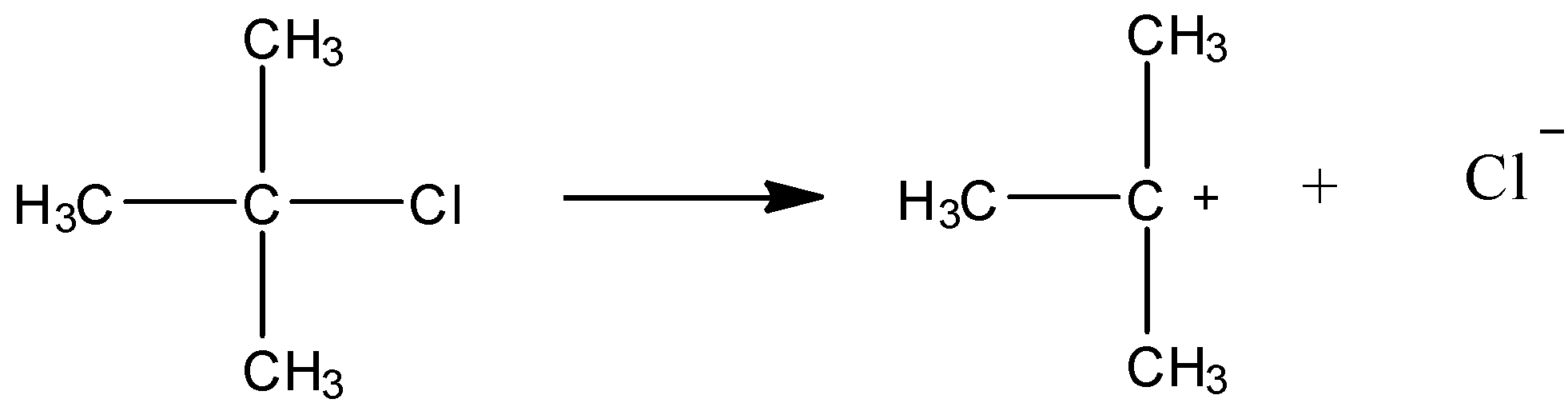
As shown in the above reaction, this compound can give chloride ions. This happens because it forms a tertiary carbocation. We know that tertiary carbocation is most stable. So, the reaction shown above will be favoured. Now, the chloride ion produced will react with Silver nitrate as shown below.
Cl−+AgNO3→AgCl+NO3−
Thus, we can say that the compound given in option (A) will give precipitates with silver nitrate solution.
B) - CHCl3 is a covalent compound. Here, three chlorine atoms are bonded with one carbon atom. Here, removal of one or more chlorine atom is not possible as the resultant species will not be stable. Thus, it cannot give precipitates with silver nitrate solution.
C) - CH2=CH−Cl is a compound having chlorine atoms. But here, chlorine is not removable as it will form a carbocation on sp2 hybridized carbon which is not stable at all. So, it will also not give precipitates with silver nitrate solution.
D) - CCl4 is a similar compound as CHCl3. It also cannot give free chlorine ions as the resultant species formed will not be stable.
So, the correct answer is “Option A”.
Note: Note that AgNO3 will form precipitates with any halogen present in the solution. So, if bromine or iodine is present in place of chlorine in a compound given in option(A), then also precipitates would have been formed.
