Question
Question: Which one of the following is ethyl - 4 (dimethylamino) butanoate? A. butanoate?
A.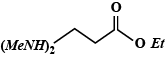
B.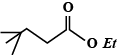
C.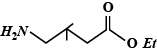
D.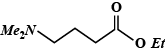
Solution
Esters are named as if the alkyl chain from the alcohol is a substituent. No number is assigned to this alkyl chain. This is followed by the name of the parent chain from the carboxylic acid part of the ester with an −e removed and replaced with the ending −oate.
Complete answer:
Esters are alkyl alkanoates. Esters are formed through reactions between an acid and an alcohol with the elimination of water. The name of an ester consists of two words. The first part of the name is the alkyl group of the alcohol and the second part of the name is the name of the acid minus the ending –ic acid plus the ending –ate.
For naming an ester, we have to follow following rules:
First we will identify the oxygen that is part of the continuous chain and bonded to carbon on both sides. (On one side of this oxygen there will be a carbonyl present but on the other side there won't be.)
Second, we will begin with numbering the carbon chains on either side of the oxygen identified in step 1.
Next, we will use this format - [alkyl on side further from the carbonyl] [alkane on the side with the carbonyl] - (In this case, it is [methyl] [methane])
And finally, we will change the ending of the alkane on the same side as the carbonyl from -e to −oate. (In this case, it is methyl methanoate)
So, the Dimethylamino group is attached to fourth Carbon in the main chain and an Ethyl group is attached to 'O' of the main chain. Hence, ethyl - 4 (dimethylamino) butanoate is:
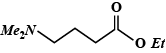
So, the correct answer is option (D).
Note:
Ethyl - 4 (dimethylamino) butanoate is also known as Ethyl 4-(dimethylamino)butyrate. Its molecular formula is C8H17NO2 and its molecular weight is 159.23 g/mol.
