Question
Question: Which one of the following Is a weak acid? A.Acetic acid B.Formic acid C.Carbonic acid D.All...
Which one of the following Is a weak acid?
A.Acetic acid
B.Formic acid
C.Carbonic acid
D.All of the above
Solution
When acids are placed in an aqueous medium or are interacted with basic substances, they tend to release a hydrogen atom from the molecule of the acid. The degree and ease to which this hydrogen atom gets released from the molecule of the acid determines the acidity of the acid. The higher the dissociation of the hydrogen atom, the higher is the acidity of that particular acid.
Complete step by step answer:
When comparing the acidity of compounds using their molecular structures, we must understand that the molecules that are more stable, tend to release their hydrogen atoms with much more ease as compared to non – stable atoms. Hence, the acidity of stable molecules is higher than non – stable molecules.
The given options to us are acetic acid, formic acid and carbonic acid. The molecular structures of these compounds can be drawn as follows:
Acetic acid:
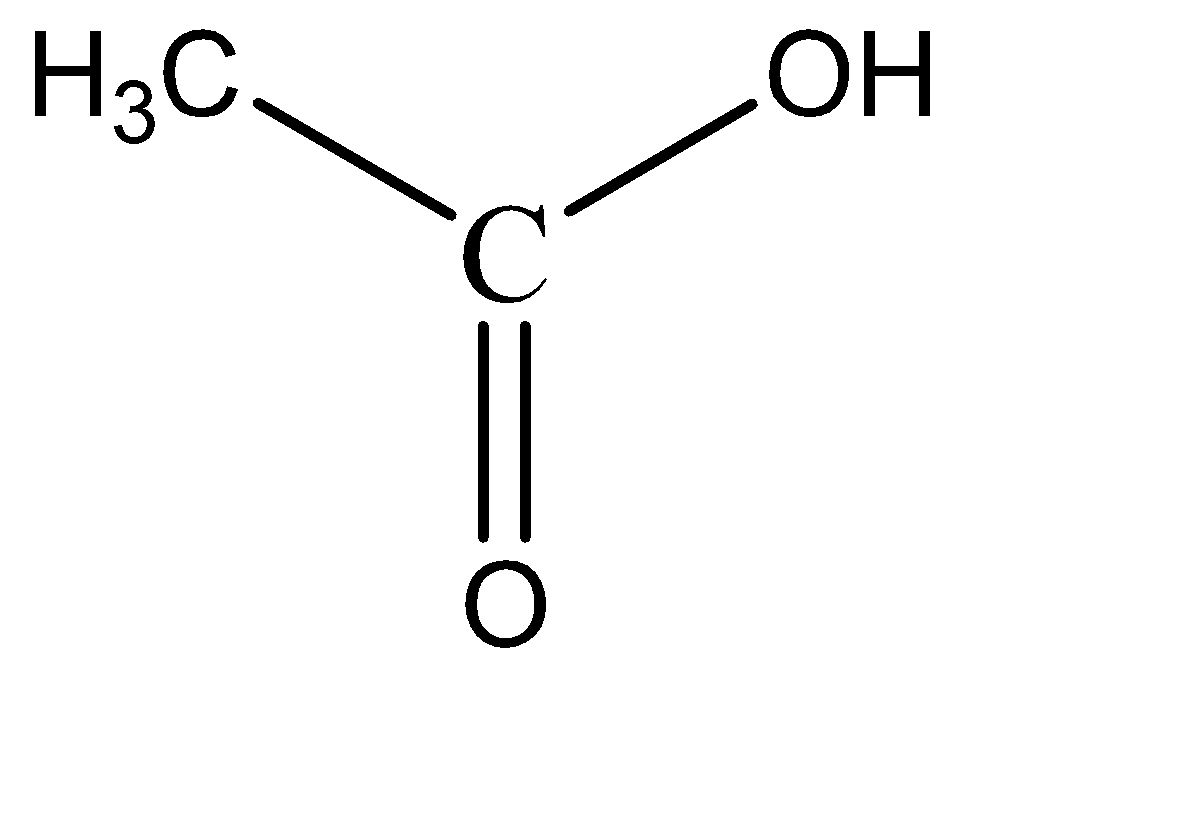
Formic acid:
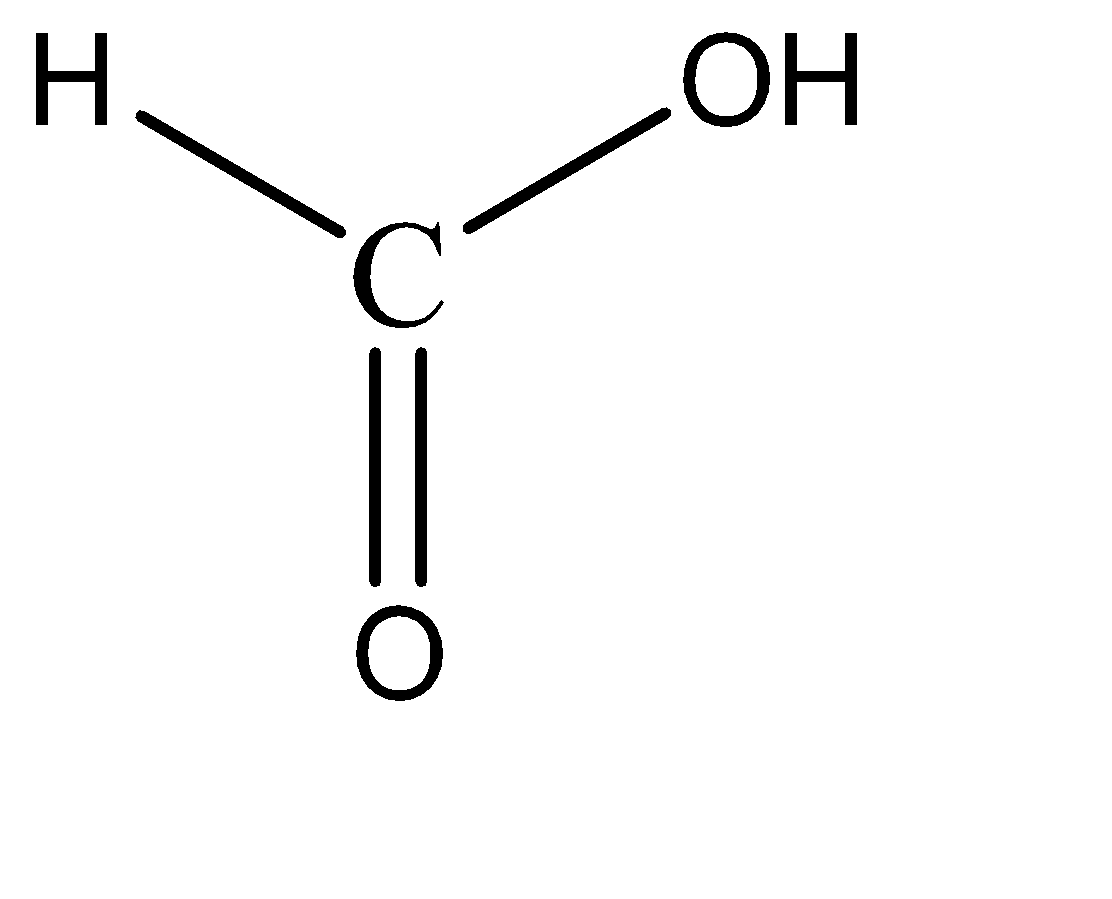
Carbonic acid:
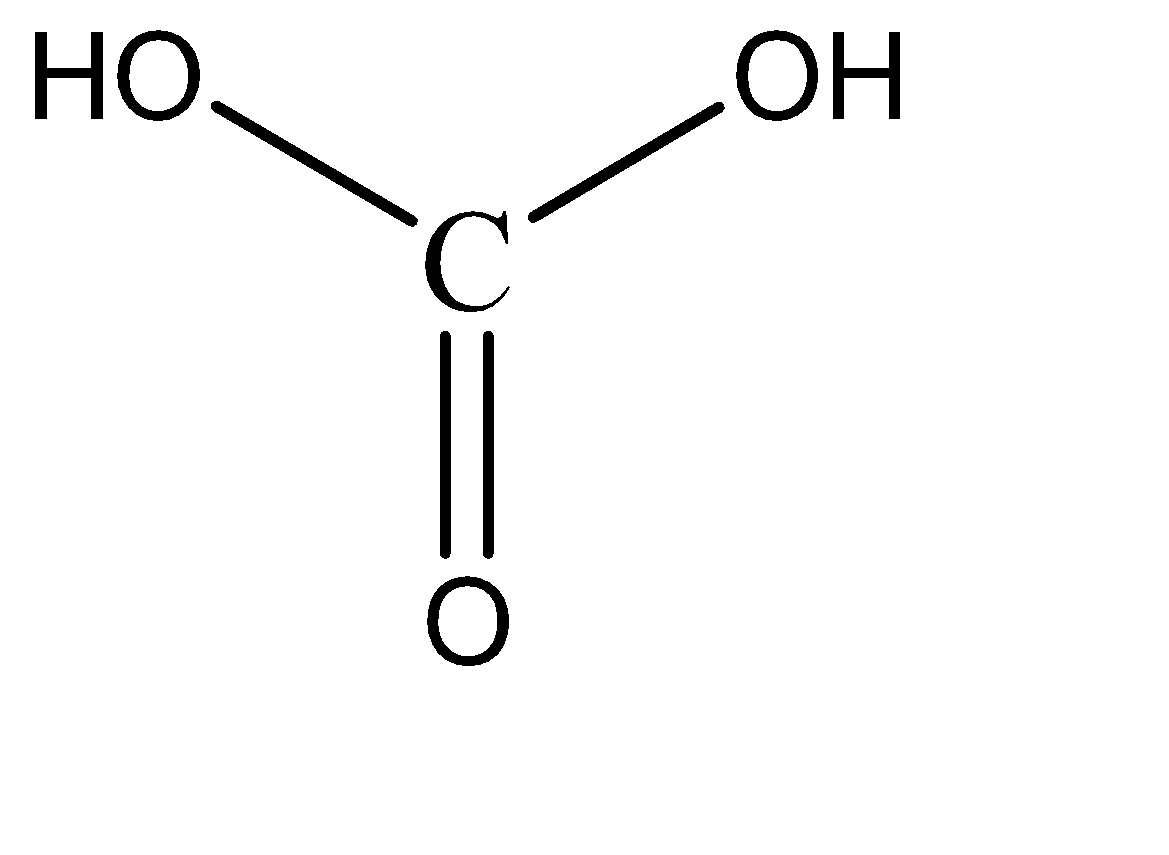
Upon observation, we can see that each option has at least one hydroxy and one carbonyl group present. These functional groups by themselves do not exhibit resonance characteristics in a compound. There is the necessity of a strongly electronegative species to initiate resonance with these functional groups.
Hence, none of these compounds have any resonance structures, and hence are relatively less stable. Hence, all these compounds are weak acids.
Hence, Option D is the correct option.
Note:
This property of acidity is mathematically measured using a method known as the pH scale. Depending on the degree of dissociation of hydrogen atoms, the acidity of the substance is calculated. On a scale of 1 to 14, substances with a pH value between 1 to 7 are known as acids, with 1 being the most acidic and 7 being the least acidic.
