Question
Question: Which one of the following is a free radical substitution reaction? A. 
B.
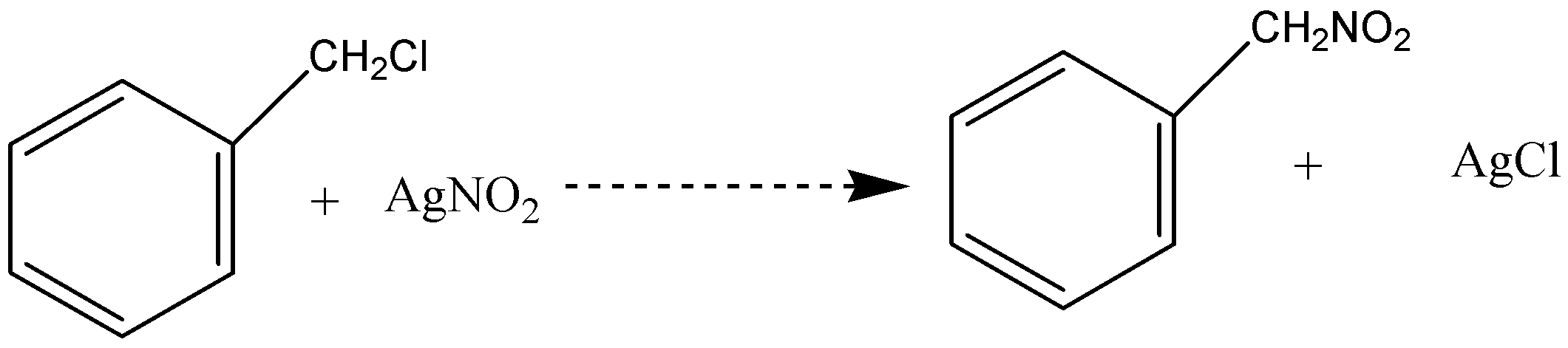
C.
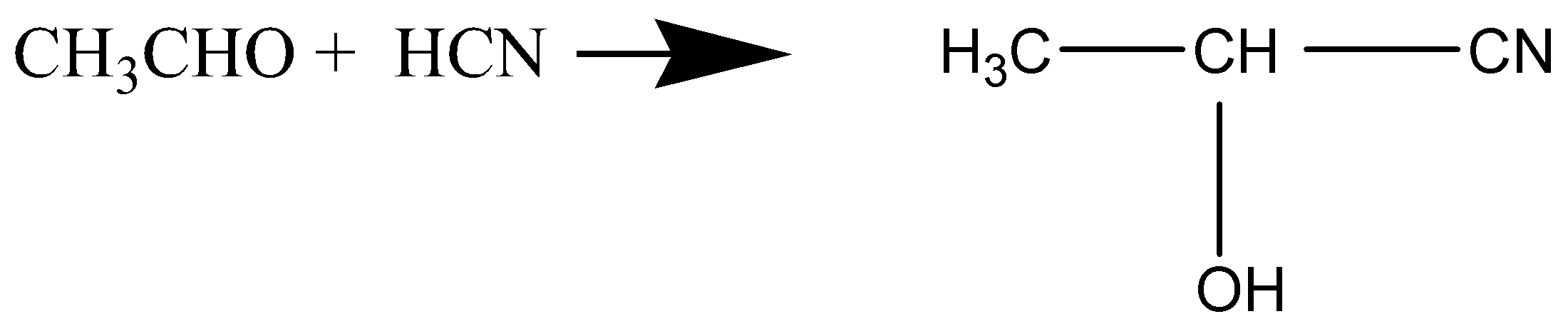
D.
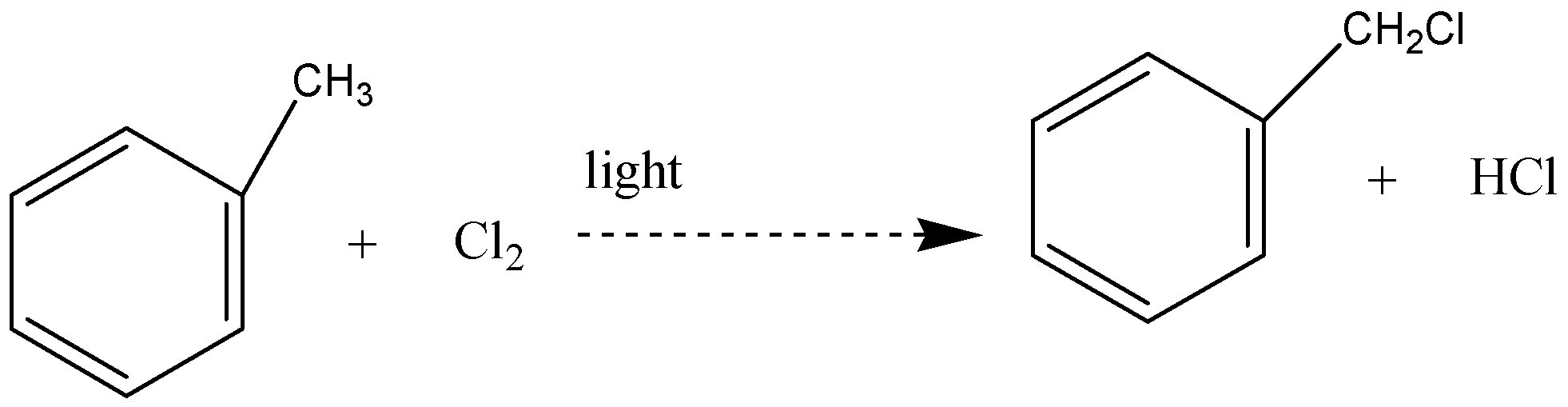
Solution
Free radical substitution reaction is the one that involves the radicals. Reaction of toluene with chlorine in presence of light is one of the examples of free radical substitution reaction. Hydrogen atoms from side chain get replaced by chlorine
Complete step by step answer:
Free radicals are atoms or groups of atoms that have a single unpaired electron and a free radical substitution reaction is the one involving these radicals. Free radicals are formed if a bond splits evenly - each atom getting one of the two electrons. The name given to this is homolytic fission.
To show that a species (either an atom or a group of atoms) is a free radical, the symbol is written with a dot attached to show the unpaired electron.
The reaction between methylbenzene and chlorine in the presence of light – typically sunlight is a free radical substitution reaction. The organic product is (chloromethyl) benzene. One of the atoms in the methyl group is replaced by a chlorine atom, so this is a substitution reaction. And all the three hydrogens in the methyl group can in turn be replaced by chlorine atoms.
Initiation Phase: This phase is initiation phase in which free radical specie is created
The chain is initiated by UV light breaking a Chlorine (Cl2) molecule into free radicals.
Cl2energysunlight2C.l
Propagation Phase:
In this, the reaction keeps the chain going
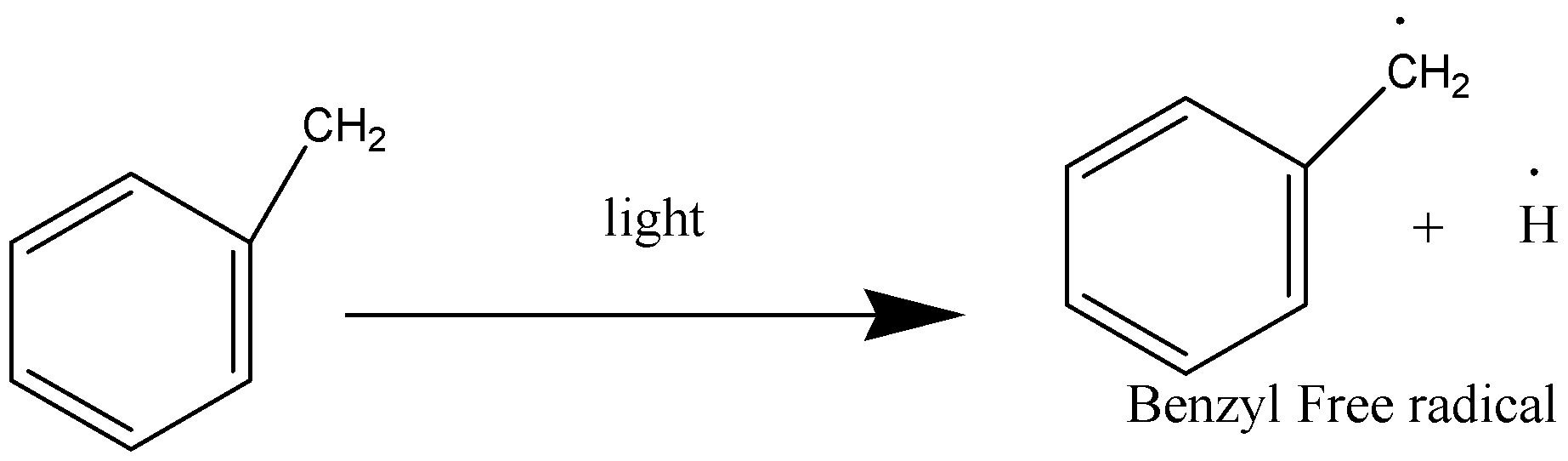
Termination Phase:
These are reactions which remove free radicals from the system without replacing them with new ones. If any two free radicals collide, they will join together without producing any new radicals.
In this reaction, hydrogen atoms from the side chain get replaced by chlorine. On monochlorination, benzyl chloride is formed on dichlorination benzal chloride
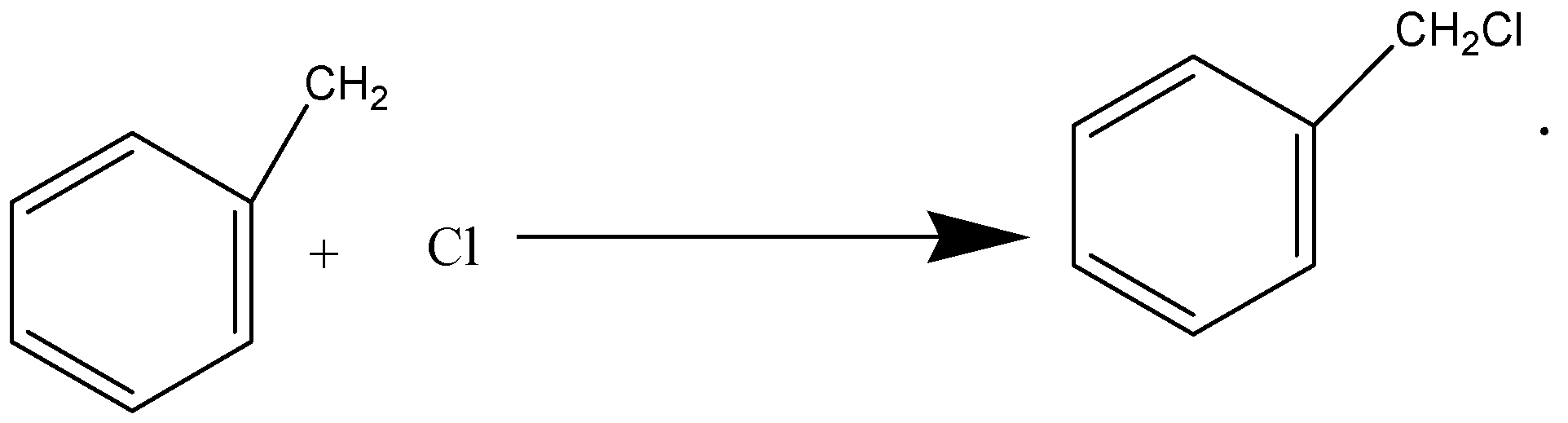
Whereas in option (A), when benzene reacts with CH3Cl in presence of anhydrous AlCl3 to give Toluene, it is fried crafts alkylation and electrophilic substitution. In option (C), when acetaldehyde reacts with hydrogen cyanide, nucleophilic attack takes place by cyanide ion to give 2-hydroxypropanenitrile as a product.
So, the correct answer is option (D).
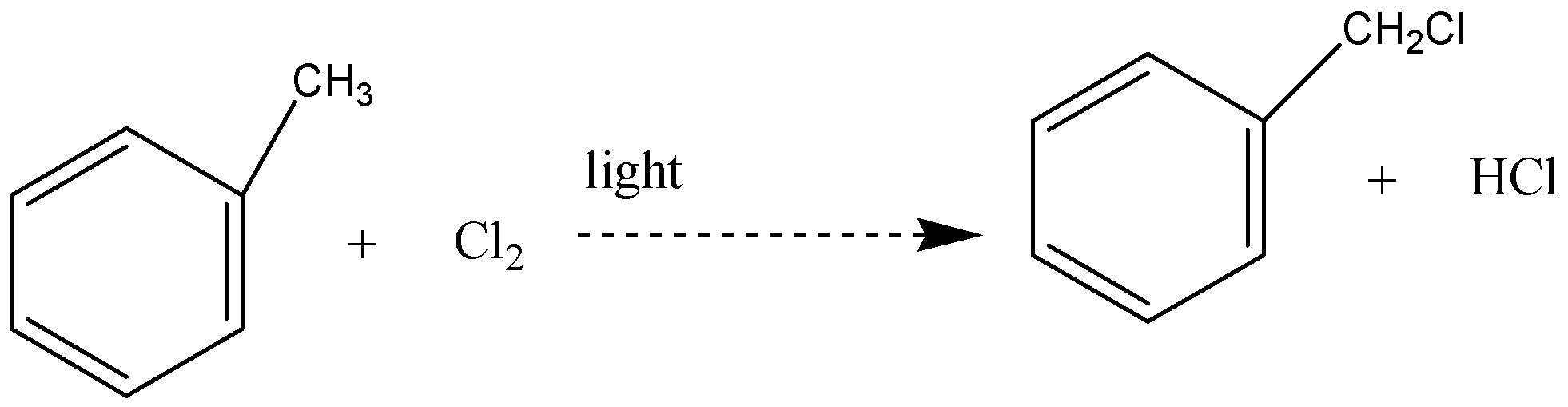
Note: Free radicals have the potential to be both extremely powerful chemical tools and extremely harmful contaminants due to their high reactivity. Most of the power of free radical species stems from the natural tendency of radical processes to occur in a chain reaction fashion Radical chain reaction has three different phases that are initiation, propagation and termination.
