Question
Question: Which one of the following has the highest dipole moment? A) \(C{{H}_{2}}C{{l}_{2}}\) B) \(CHC{{...
Which one of the following has the highest dipole moment?
A) CH2Cl2
B) CHCl3
C) CCl4
Solution
The dipole moment of the polar compounds is other than zero, and non-polar compounds have dipole moment zero.
If a molecule has the same atoms linearly present in front of each other, attached to the central atom, having the same magnitude but opposite direction of dipole, then their dipole moments will cancel out and the net dipole moment will be zero.
Complete step by step answer:
In order to compare the dipole moments of the given molecules we must first understand the concept of dipole moment.
Dipole moment is a phenomena that occurs when there is a charge separation. Dipole moments can be observed between two different ions with ionic bonds or it could also be observed between atoms which are attached to each other through covalent bonds; dipole moments come into play because of the differences in electronegativity. And with the increase in difference in electronegativity, the dipole moment also increases. The distance which is present between the positive and negative charge separation is also responsible for the size of the dipole moment. The value of dipole moment is a measure of the polarity which is present in the molecule.
Now, we will look at every option given in the question, and draw their Lewis dot structures, in order to understand the dipole moments. If we consider the first option it says dichloromethane, so the Lewis dot structure of dichloromethane is shown below,
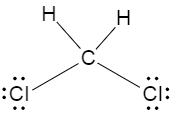
Now if we consider the second option, two of the chlorine groups are in front of each other, meaning they cancel out each other’s dipole moment, and the net dipole moment decreases as a result.
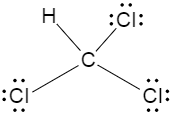
Now if we consider the third option, the Lewis dot structure of carbon tetrachloride is drawn below,
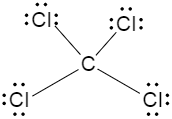
Now, we can see that each of the chlorine atoms are located in front of the other chlorine molecule, in other words, the dipole moments of the chlorine atom, cancels each other out because they are of same magnitude but in opposite directions. So, we can say that the carbon tetrachloride is a nonpolar molecule.
So, after comparison of the three structures, we can see that the net dipole moment of the dichloromethane is the highest among the three.
Hence, the correct answer is Option (A) .
Note: The dipole moment of dichloromethane is highest among trichloromethane and carbon tetrachloride, because, in trichloromethane two of the chlorine are located in front of each other having same magnitude but opposite direction of dipole moment, so they cancel out each other’s dipole moment resulting in lesser net dipole moment. And carbon tetrachloride is a non-polar molecule because all four chlorine atoms cancel out the dipole moment of each other resulting in zero net dipole moment.
