Question
Question: Which one of the following has the highest boiling point? A. \({H_3}CC{H_2}C{H_2}C{H_2}Cl\) B. ...
Which one of the following has the highest boiling point?
A. H3CCH2CH2CH2Cl
B. (H3C)2CHCH2Cl
C. (H3C)3CCl
D. H3CCH2CH(Cl)CH3
Solution
Let us identify the above hydrocarbon chains. Since each one of them has a chloride in place of hydrogen such compounds are alkyl halides. We know in the general cases the boiling point of the unbranched alkanes show a regular increase with an increase in the molecular weight.
Complete step by step solution:
Let us now understand the reasons that affect the boiling point of the compounds. So with the unbranched alkanes, as molecular weight increases, so do the molecular size and surface area. With increasing surface area, the van der waals forces between the molecules increase; therefore more energy or a higher temperature will be required to separate molecules from one another and produce boiling .
Chain branching on the other hand makes a molecule more compact, reducing its surface area and with the strength of the vander waals forces operating between it and adjacent molecules which has the effect of lowering the boiling point.
Now let us analyse each alkyl halide one by one, the first one is butyl chloride as it is a four carbon chain and has a chloride attached to it. It is a simple unbranched alkyl halide chain. The second one is (2-methyl) propyl chloride and it is branched as we can see in the diagram. The third is (2,2 dimethyl) propyl chloride it is heavily branched as compared to the second compound and the last compound is (2-chlorobutane)
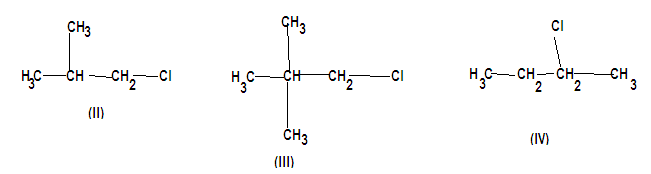
Van der waals forces for nonpolar molecules are high which is why they have a higher boiling point. But none of the above compounds are non polar. The polarizability that is an important factor to determine the magnitude of van der waals force is not very significant in chlorine. Therefore we can conclude that compound (1) has the highest boiling point from the above explanation.
**Hence the correct option is option A.
Note:**
alkyl halides are also called haloalkanes. They are classified as primary, secondary and tertiary on the basis if the halogen is attached to a primary carbon it is primary halide similarly secondary and tertiary halides can be classified the same way.
