Question
Question: Which one of the following has an O-O bond? A.\[{H_2}{S_2}{O_6}\] B.\[\;{H_2}{S_2}{O_7}\] ...
Which one of the following has an O-O bond?
A.H2S2O6
B.H2S2O7
C.H2S2O5
D.H2S2O8
Solution
We know that a peroxide linkage is simply a O−O bond. The oxygen-oxygen bond is quite weak, and the bond can hemolyze. So, we will see which of the following compounds in the question has peroxide linkage.
Complete answer:
Peroxide is any of a class of chemical compounds in which two oxygen atoms are linked together by a single covalent bond i.e. O−O bond.
Peroxide linkage (−O−O−) is present in H2S2O8 as its name also suggests peroxide linkage H2S2O8is called per-oxo disulphuric acid (Marshall' acid)
The molecular structure of oxoacids is shown in the figure below. H2S2O8 has –O - O- bond.
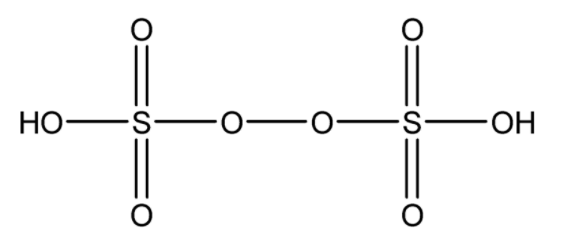
The other 3 options in the questions do not have –O - O- bond.
Therefore, the correct answer is option (D).
Note:
Marshall Acid is H2S2O8 which is also known as peroxydisulfuric acid. It is called Marshall's acid after its inventor Professor Hugh Marshall, it is a sulphur oxoacid.. It contains sulphur in its +6 oxidation state and a peroxide group. Its salts, which are commonly known as persulphates, are industrially important as the powerful oxidizing agents. This acid is prepared by the reaction of chlorosulfonic acid with hydrogen peroxide and also can be generated from potassium or ammonium persulfate in acidic solution. Peroxydisulfuric Acid is a colourless solid and it is one of the most powerful peroxyacid oxidants available. The use of peroxydisulphuric acid and its salts as a source of hydrogen peroxide opened the way for large-scale production of sulphuric acid.
