Question
Question: Which one of the following halogen compounds is difficult to be hydrated by \({{S}_{N}}1\) mechanism...
Which one of the following halogen compounds is difficult to be hydrated by SN1 mechanism?
(A) Tertiary butyl chloride
(B) Isopropyl chloride
(C) Benzyl Chloride
(D) Chlorobenzene
(E) Allyl chloride
Solution
For determining which of this halogen compound is difficult to be hydrated by SN1 mechanism we need to find out in which of them halogen atom is firmly attached and cannot be replaced by nucleophile OH− easily.
Complete step by step solution:
We have been provided with halogen compounds Tertiary butyl chloride, Isopropyl chloride, Benzyl Chloride, Chlorobenzene and Allyl chloride.
We need to find out which of the halogen compounds is difficult to be hydrated by SN1 mechanism.
So, for that:
First, we have tertiary butyl chloride:
So, tertiary butyl chloride is unstable and can easily be hydrolyzed or hydratedSN1 mechanism.
Next, we have, isopropyl chloride:
Isopropyl chloride is unstable and can easily be hydrolyzed or hydrated by SN1 mechanism.
Next, is benzyl chloride:
Benzyl chloride is unstable and can easily be hydrolyzed or hydrated by SN1 mechanism.
Next, we have, chlorobenzene:
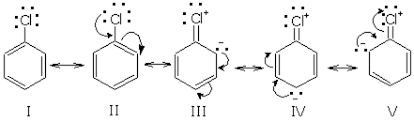
Chlorobenzene is quite stable due to the delocalization of electrons by resonance. Also, (C-Cl) bonds possess a double bond character due to which it is very difficult to hydrolyze by SN1 mechanism.
Last one is Allyl chloride:
Allyl chloride is unstable and can easily be hydrolyzed or hydrated by SN1 mechanism. So, we can say that chlorobenzene is very difficult to hydrolyze by SN1 mechanism.
Therefore, we can conclude that option (D) is correct.
Note: The stability of resonance increases with the number of covalent bonds. Number of atoms with an octet of electrons. A negative charge if any on a more electronegative atom, a positive charge if any on the more electropositive atom, increases the stability of the atom.
