Question
Question: Which one of the following exhibits the largest number of oxidation states? (a) Ti(22) (b) V(23)...
Which one of the following exhibits the largest number of oxidation states?
(a) Ti(22)
(b) V(23)
(c) Cr (24)
(d) Mn(25)
Solution
The oxidation number of an atom in a species is the total number of electrons it gains or loses while forming a bond with another atom present in the species. An element can exhibit different oxidation states in different species. We can theoretically calculate the oxidation state of an atom in a species which may or may not be equal to the observed oxidation state of that atom in the species.
Complete answer:
For solving this question, we first need to understand the meaning of oxidation state. The oxidation number of an atom in a species is the total number of electrons it gains or loses while forming a bond with another atom present in the species. There are two types of oxidation numbers. (a) Calculated or average oxidation number and (b) observed oxidation number. Sometimes the values for both the calculated oxidation number and the observed oxidation number are the same. But they are different for some species. For such species we have to find the actual oxidation number by using the chemical structure of the species.
Calculated oxidation number:
For finding the calculated oxidation number of a particular atom in a species, we have to assume the oxidation number of all other atoms present in the species. The valence number of the atoms with their sign is taken as their assumed oxidation number. For example, if we have to find the oxidation number of hydrogen in H2O, we will assume the oxidation number of oxygen as -2 where 2 is the valency of O atom. Therefore the oxidation number of H will be:
Sum of all the atoms present in the species = overall charge present on the species
Let the oxidation number of H be x,
2x−2=0 (since there are two atoms of hydrogen present in the molecule, therefore x is multiplied by 2.Also the overall charge on the molecule is zero)
x=+1
Observed oxidation number:
For calculating observed oxidation numbers we first need to draw the chemical structure of the species.
This is the structure of HSO3−.
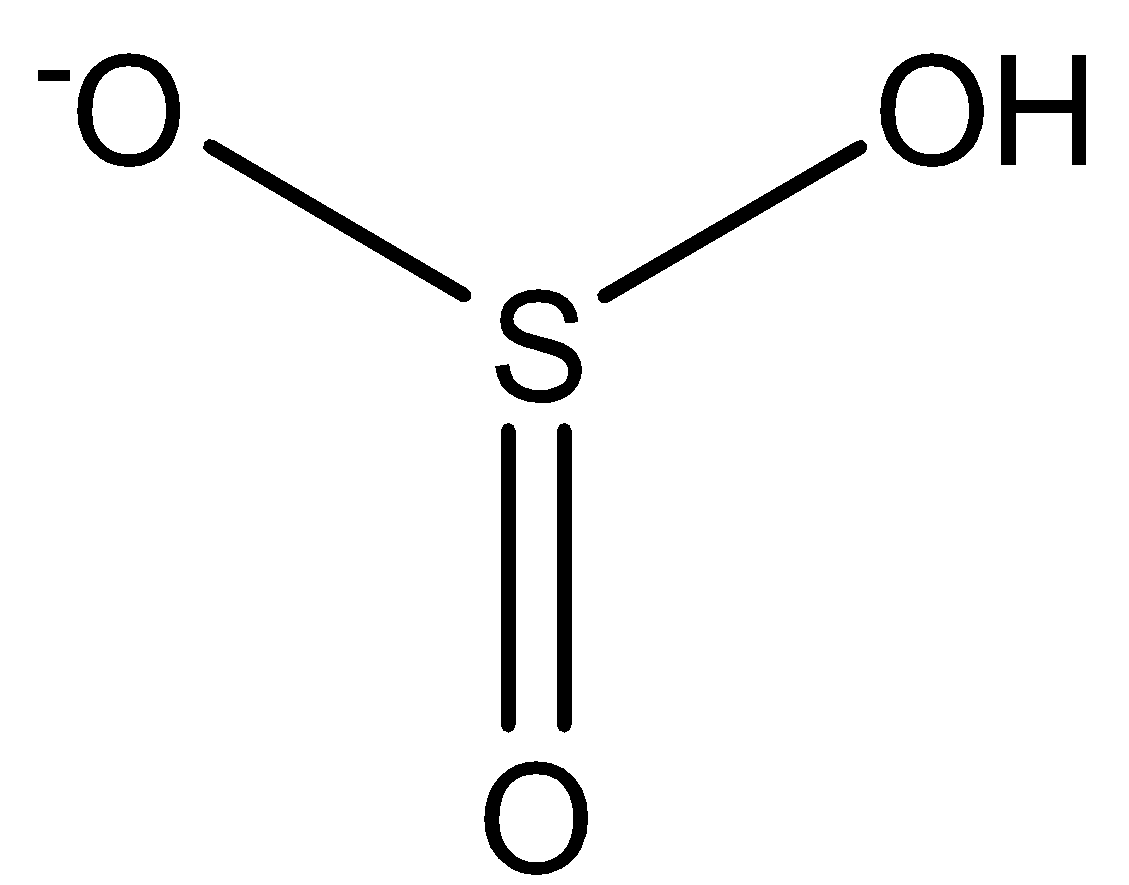
Now, a single bond comprises two electrons, a double bond comprises 4 electrons and a triple bond comprises 6 electrons. So if there is a single bond between the same atoms then the electrons are equally shared between them such that the oxidation number on each atom will be 0. But if is between two different types of atom then the more electronegative atom will attract both of the electrons present in the single bond towards itself such that the oxidation number of the more electronegative atom will be -1 while that of the less electronegative atom will be +1. Since O is more electronegative than S, therefore it will attract all the bonded electrons towards itself. Hence the oxidation number of S in HSO3− is +4.
Now, let us solve the question. An element can show different oxidation states in different species. The more unpaired electrons an element has in its ground state, the more varying oxidation states it can exhibit when present in different species. Let us look at the electronic configurations of each element given to us:
Ti has an electronic configuration: [Ar]3d24s2. Thus it has two unpaired electrons in its 3d sub-shell and two paired electrons in 4s sub-shell. Hence the maximum oxidation state that it can show is +4 and the minimum oxidation state that it can show is +2. The general oxidation states (stable oxidation states) of Ti in its compounds are +2, +3 and +4.
V has an electronic configuration: [Ar]3d34s2. Thus it has three unpaired electrons in its 3d sub-shell and two paired electrons in 4s sub-shell. Hence the maximum oxidation state that it can show is +5 and the minimum oxidation state that it can show is +2. The general oxidation states (stable oxidation states) of V in its compounds are +2, +3, +4 and +5.
Cr has an electronic configuration: [Ar]3d54s1. Thus it has five unpaired electrons in its 3d sub-shell and one un-paired electron in 4s sub-shell. Hence the maximum oxidation state that it can show is +6 and the minimum oxidation state that it can show is +1. The general oxidation states (stable oxidation states) of Cr in its compounds are +6, +3 and +2.
Mn has an electronic configuration: [Ar]3d54s2. Thus it has five unpaired electrons in its 3d sub-shell and two paired electrons in 4s sub-shell. Hence the maximum oxidation state that it can show is +7 and the minimum oxidation state that it can show is +2. The general oxidation states (stable oxidation states) of Mn in its compounds are +2, +3, +4, +5, +6 and +7.
Hence the correct answer is (d) Mn(25).
Note:
Do not get confused between valency and oxidation state for an element. The valency or valence of an element is defined as its combining power when it combines which other atoms or a group of atoms in a compound. This combining power is determined by the number of hydrogen atoms that it combines with. For example carbon has a valency of 4 since it combines with four hydrogen atoms to give methane. The elements that have one, two, three or four electrons in their outermost shell or valence shell, they have the valency of one, two, three and four respectively. For elements having more than 4 electrons in their valence shell, their valency can be easily found by using the formula:
Valency = 8-number of electrons in the valence shell.
However, for transition metals we generally do not use the term valency but rather look at their oxidation states in their compounds since for transition metals we consider the electrons present in the (n-1)d sub-shell as part of the valence electrons due to which they can combine with different elements in different ratios because of the presence of more unpaired electrons.
