Question
Question: Which one of the following esters gets hydrolyzed most easily under alkaline conditions? 
Solution
The substituents –H, -Cl, −NO2 and −OCH3 are neutral, electron donating, electron withdrawing and electron donating respectively. The compound which has the substituent that makes the carbonyl carbon most electrophilic will get hydrolyzed easily.
Complete step by step solution:
Let’s see the mechanism of the alkaline hydrolysis of esters in order to find which one of the given esters will undergo hydrolysis easily.
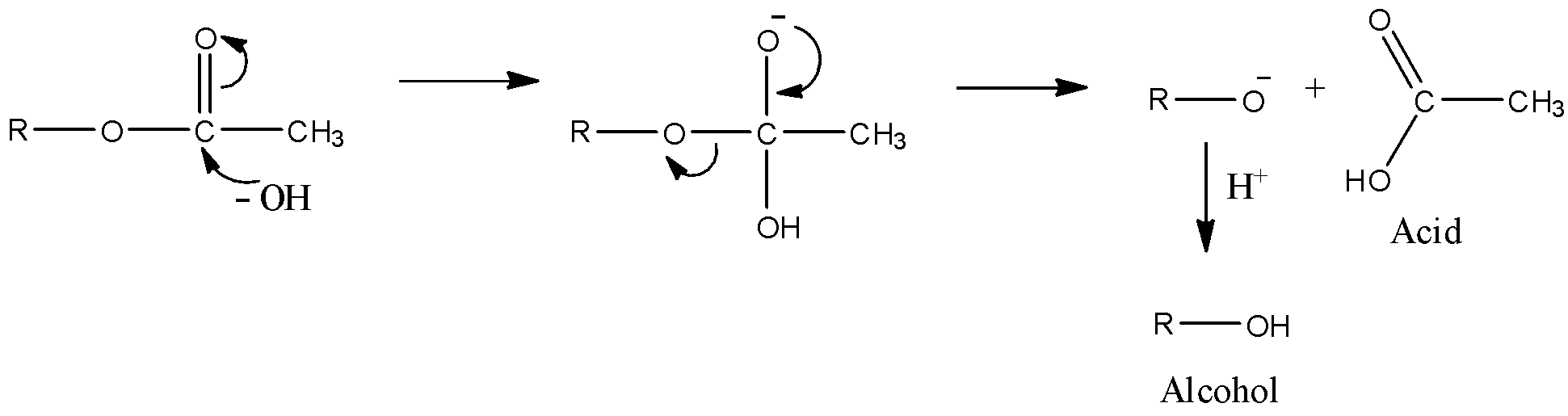
Here, we can see that the first step is the attack of the hydroxyl ion of the electrophilic carbon of the carbonyl group. Then, the alkoxy group leaves the molecule and they react with protons in order to form alcohol. An acid is also formed. Thus, upon hydrolysis of ester, alcohol and acid is formed.
- Now, in order to find which ester would get hydrolyzed easily, we need to see the first step. It will decide which acid will react more easily.
- We can say that if the carbonyl group is more electrophilic, then it would be more susceptible to the nucleophilic attack.
- We have four compounds in which substituents at para positions differ.
- Hydrogen atoms do not have any effect on the carbonyl carbon. Chlorine atoms at para position will increase the electron density on carbonyl carbon. So, it will also not facilitate the nucleophilic attack.
- Nitro group is a strong electron withdrawing group. So, it will make the carbonyl carbon most electrophilic. Thus, we obtain that this ester will be easier to hydrolyze. Methoxy group being an electron donating group will increase the electron density and thus, the electron density on carbon will increase.
Thus, we can say that compound in option (C) will be easily hydrolyzed. So, this is the correct answer.
Note: Note that chlorine and other halogen atoms can donate the electron density via resonance. However, they have high electronegativity, so they can accept electron density via inductive effect.
