Question
Question: Which one of the following does not follow the octet rule: \(A.\,\,P{F_3}\) \(B.\,\,B{F_3}\) ...
Which one of the following does not follow the octet rule:
A.PF3
B.BF3
C.CO2
D.CCl4
Solution
The octet rule is a chemical rule of thumb that tends to bond in such a way that each atom has eight electrons in its valence shell which results in giving the stable electronic configuration. The valence electrons can be counted using a Lewis electron dot diagram for the compounds. A Lewis electron dot diagram is a representation of the valence electrons of an atom that uses dots around the symbol of the element. As we know that the number of dots will be equal to the number of valence electrons in an atom.
Complete step-by-step answer: Let’s observe the options and see how it approaches the Lewis structure;
In the first option PF3 , let’s see the Lewis structure;
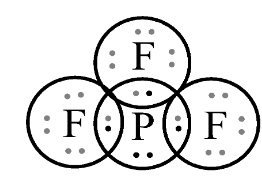
The number of electrons in the last shell of phosphorus is 8 . Since, the valance electrons are 5 in which it combines with fluorine, which results in one lone pair and three bond pair of electrons with fluorine which tends to follows octet rule.
In second option BF3 , Let’s draw the structure of BF3 ;
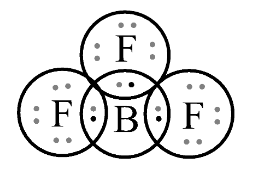
In this structure, it does not follow octet rule because central boron atom is surrounded by 6 electrons. The 3 electrons are of Boron atom and remaining 3 electrons are of 3 fluorine atoms. So, to satisfy octet rule, there is in need 8 electrons which follow the stable structure. As we know, Boron lacks an electron pair. Hence, it also acts as Lewis acid.
In third optionCO2, let’s see the Lewis structure;
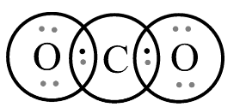
By observing the structure, we can see that it is following the octet rule. The central atom, Carbon satisfies its octet by sharing 2 pairs of electrons from two oxygen atoms, in addition to its valence electrons. Each of these molecules has 8 electrons on the central atom which satisfies their stable electronic configuration.
In fourth option CCl4 , let’s see the Lewis structure;
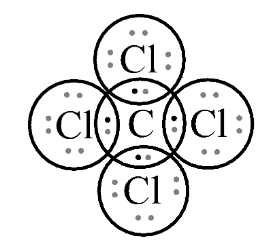
In this, we can observe again that it is following the octet rule. A single covalent bond attaches each of the four chlorine atoms to a single central carbon atom. Carbon has four valence electrons where it shares with each chlorine to form a stable compound.
So, in these given options we can observe that BF3 is unable to fulfil the octet rule. So, the answer is BF3 .
Therefore, the correct option is B.BF3 .
Note: The octet rule states that atoms with an atomic number tend to combine so that they each have eight electrons in their valence shells, which give them the same electronic configuration as a noble gas.
The two elements that most commonly fail to complete an octet are boron and aluminium; they both readily form compounds in which they have six valence electrons, rather than the usual eight predicted by the octet rule.
