Question
Question: Which one of the following does not have \(s{p^2}\) hybridised carbon? A....
Which one of the following does not have sp2 hybridised carbon?
A.Acetone
B.Acetic Acid
C.Acetonitrile
D.Acetamide
Solution
In this question, we need to determine that of the given options which compound does not have sp2 hybridised carbon. For this, we will first draw the structure of each of the given options and then back bonding form. Hybridisation can easily be recognized by seeing the structure of the compound.
Complete step-by-step answer:
Let’s see the (a) acetone structure,
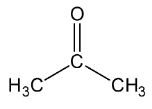
In C=O, the C is sp2 hybridised.
In both CH3, theC−H single bound therefore the C is sp3 hybridised.
Let’s see the (b) acetic acid structure,
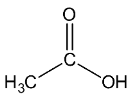
In C=O, the C is sp2 hybridised.
In both CH3, the C−H single bound therefore the C is sp3 hybridised.
Let’s see the (c) acetonitrile structure,
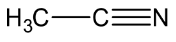
In C≡N, the C is sp hybridised .
In both CH3, the C−H single bound therefore the C is sp3 hybridised.
Let’s see the (d) acetamide structure,
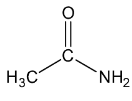
In C=O, the C is sp2 hybridised .
In both CH3, the C−H single bound therefore the C is sp3 hybridised .
Therefore, (C) is the correct answer. Because the (c) option doesn’t have sp2 hybridised Carbon.
Note: Remember to draw structures and make sure that common names and IUPAC names should be known. Nitrogen can form triple and in ionic form it forms four bonds. In this question the acetonitrile was not showing any sp2 hybridised Carbon because of the structure. sp2 carbon shows oneσ−twoπ bonds, whereas in acetonitrile’s case we are able to observe that it shows sp hybridised oneσ−twoπ bonds.
