Question
Question: Which one of the following cycloalkane gives an open chain compound when it reacts with bromine? A...
Which one of the following cycloalkane gives an open chain compound when it reacts with bromine?
A.Cyclopropane
B.Cyclopentane
C.Cyclohexane
D.Cyclo-octane
Solution
(1) Alicyclic hydrocarbons contain rings or closed chains of carbon atoms in their molecules and have properties similar to those of the open chain or acyclic hydrocarbons. There are two categories in which alicyclic hydrocarbons are divided: saturated and unsaturated alicyclic hydrocarbons. The saturated alicyclic hydrocarbons are called cycloalkanes.
(2) Cycloalkane rings which are smaller in size are more susceptible to suffer from ring strain.
Complete step by step answer:
Ring strain in ring compounds is a kind of instability that occurs when bonds in a molecule form abnormal angles.
Although each carbon in cycloalkane is bonded to two carbons and two hydrogens, the bond angles are not the ideal 109.5o of tetrahedral sp3 carbon because of the limitations of cyclic structure. The smaller rings have bond angles smaller than the tetrahedral 109.5o . They have high bond energy and attain more p-character to reduce this energy. So, they suffer from very high ring strain.
Among cycloalkanes, cyclopropane is the smallest in ring size and hence it suffers from ring strain more than the other alkanes. The bond angles in cyclopropane are 60o rather than 109.5o. Thus, the extreme angle strain leads to overlap of the sp3 orbitals and there is repulsion between the bonding pairs. Because of this instability of the bond, cyclopropane is more reactive than the other alkanes. It has the tendency to attain stability by breaking the ring.
When cyclopropane reacts with bromine, it undergoes an addition reaction in which the ring is broken to give an open chain compound. In this way, the system becomes more stable.
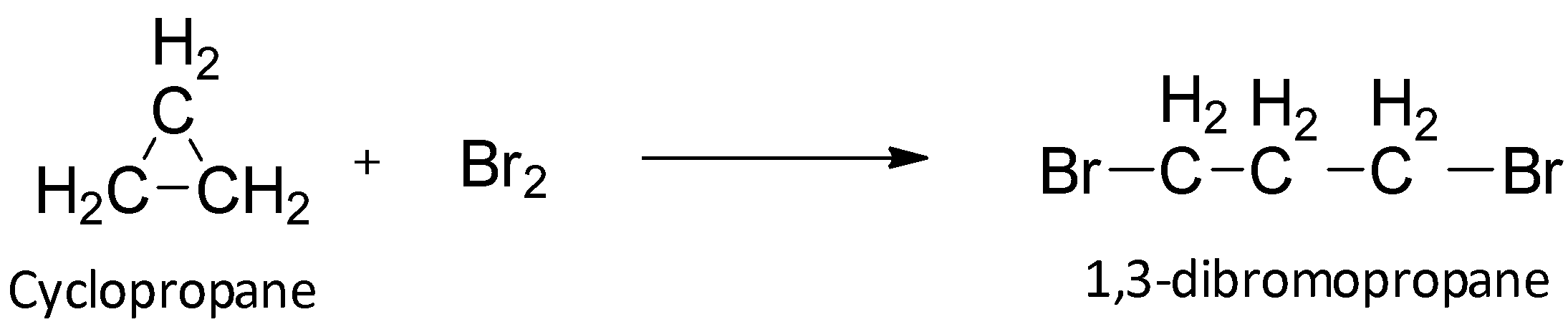
So, option A is correct.
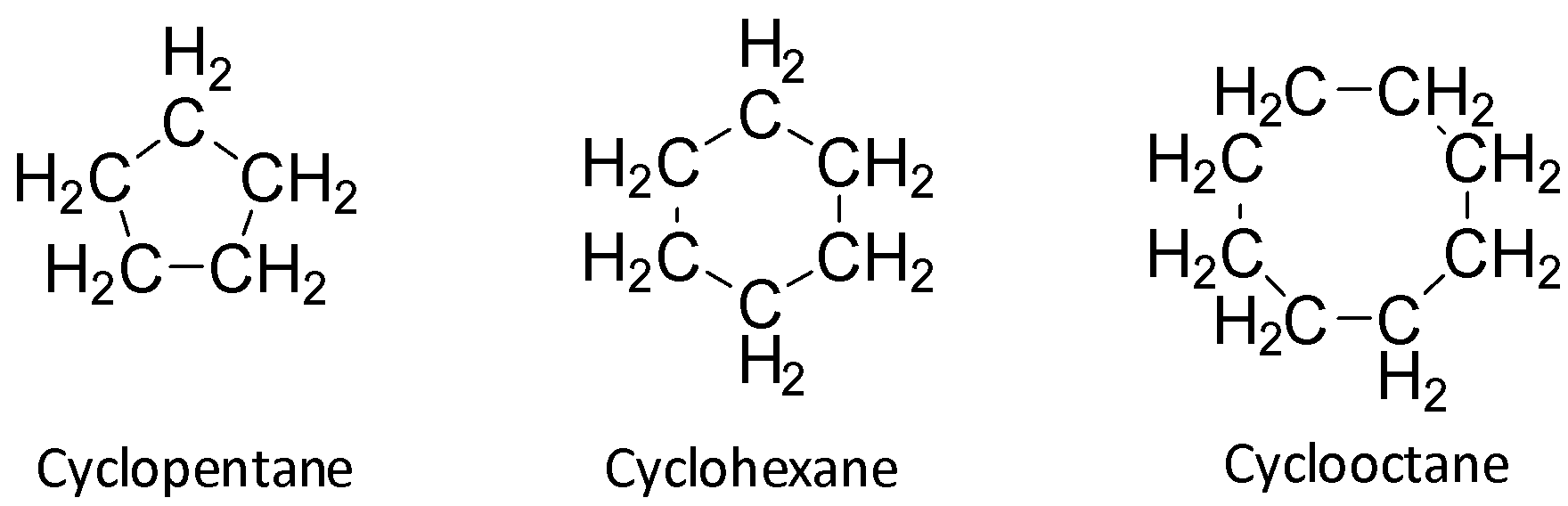
Cyclopentane, cyclohexane and cyclo-octane have ring sizes greater than that of cyclopropane and so they suffer from less strain as compared to cyclopropane.
So, they do not have the tendency to form an open chain compound. So, B, C and D are wrong.
Note:
Another small sized cycloalkane is cyclobutane. It also suffers from ring strain. Here, the bond angle is found to be 88o as it has a slightly folded form to relieve the torsional strain. The intense strain of cyclobutane is mainly because of angle strain.