Question
Question: Which one of the following compounds is not optically active? (A) \(C{{H}_{3}}C{{H}_{2}}CH(C{{H}_{...
Which one of the following compounds is not optically active?
(A) CH3CH2CH(CH3)CH2Cl
(B) CH3CH2CH(CH3)2
(C) CH3−CHOH−COOH
(D) CH3−CHCl−CH2Br
Solution
Optical activity is only shown by chiral molecules. So, to solve this question we first need to know what chiral molecules are. A molecule that has a non-superimposable mirror image at any combination of translations and rotations is known as a chiral molecule.
Complete answer:
To determine which molecule is not optically active we have to determine which molecule is not chiral.
Now, in a chiral molecule, there is a presence of chiral carbon. The carbon atom that contains four different bonded groups, including lone pairs, is known as a chiral carbon.
First, let us draw the structures of each molecule.
Now, let us determine the presence of chiral atoms in each of the options
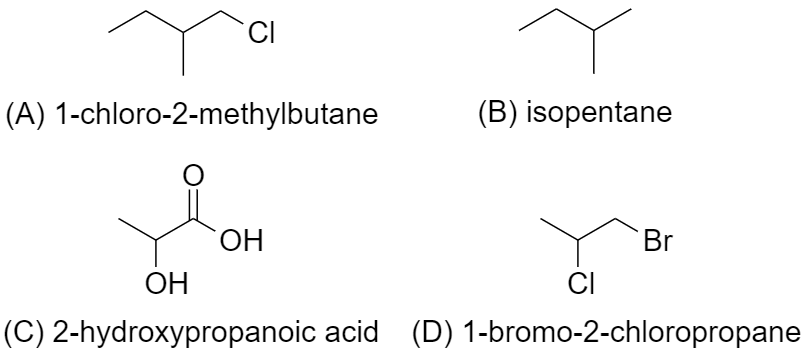
(A) In 1-Chloro-2methylbutane (CH3CH2CH(CH3)CH2Cl), there is 1 chiral carbon.
Since it has a chiral carbon, which is attached to 4 distinct bonding group, which are −CH2CH3, −CH3, -H, and −CH2Cl
Hence, it is optically active.
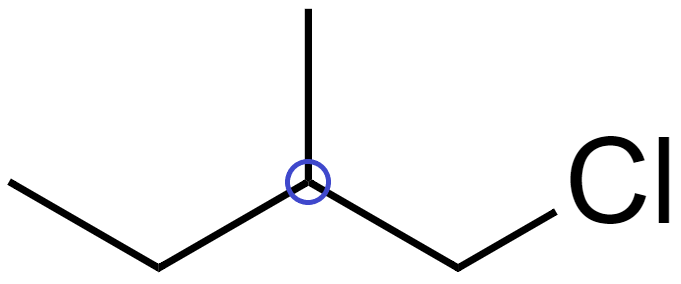
(B) In isopentane (CH3CH2CH(CH3)2), there are no chiral carbons.
Since no atom is attached to 4 distinct bonding groups, it does not have a chiral carbon and hence is not optically active.
(C) In 2-hydroxypropanoic acid (CH3−CHOH−COOH), there is 1 chiral carbon.
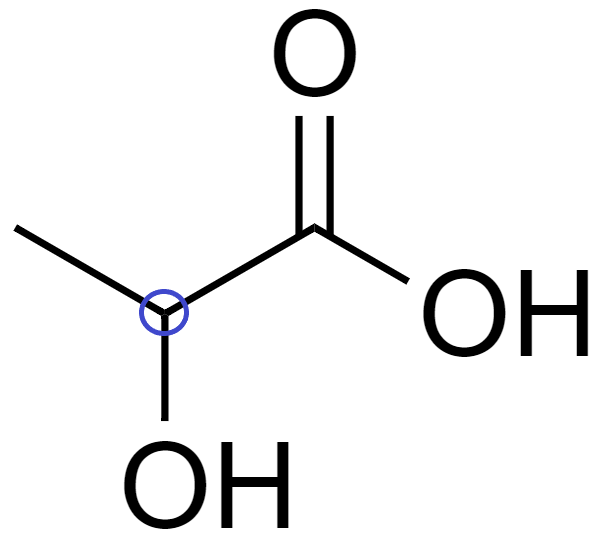
Since it has a chiral carbon, which is attached to 4 distinct bonding groups, which are −COOH, −CH3, -OH, and -H.
Hence, it is optically active.
(D) In 1-Bromo-2-chloropropane (CH3−CHCl−CH2Br), there is 1 chiral carbon.
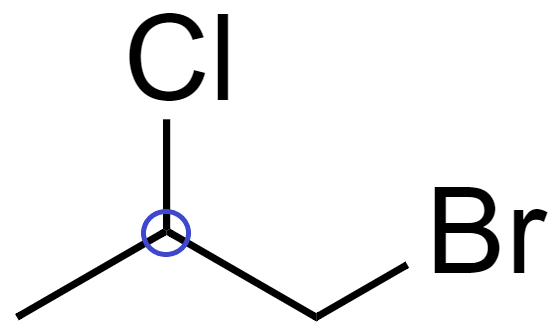
Since it has a chiral carbon, which is attached to 4 distinct bonding group which are −CH3, -Cl, -H and −CH2Br
Hence, it is optically active.
So, the correct option is option (B) CH3CH2CH(CH3)2 (isopentane).
Note:
It should be noted that an atom having 2 or more than 2 identical groups attached to it is not a chiral center.
For example, the straight-chain alkyl group's carbon atoms are not chiral, as they have multiple hydrogens attached to them (−CH2 or -CH3).
A carbon atom having multiple bonding like a double bond or triple bond is not chiral as it does not have four distinct ligands.
