Question
Question: Which one of the following compounds is most reactive for \[{E_1}\] reaction? A. 
B.
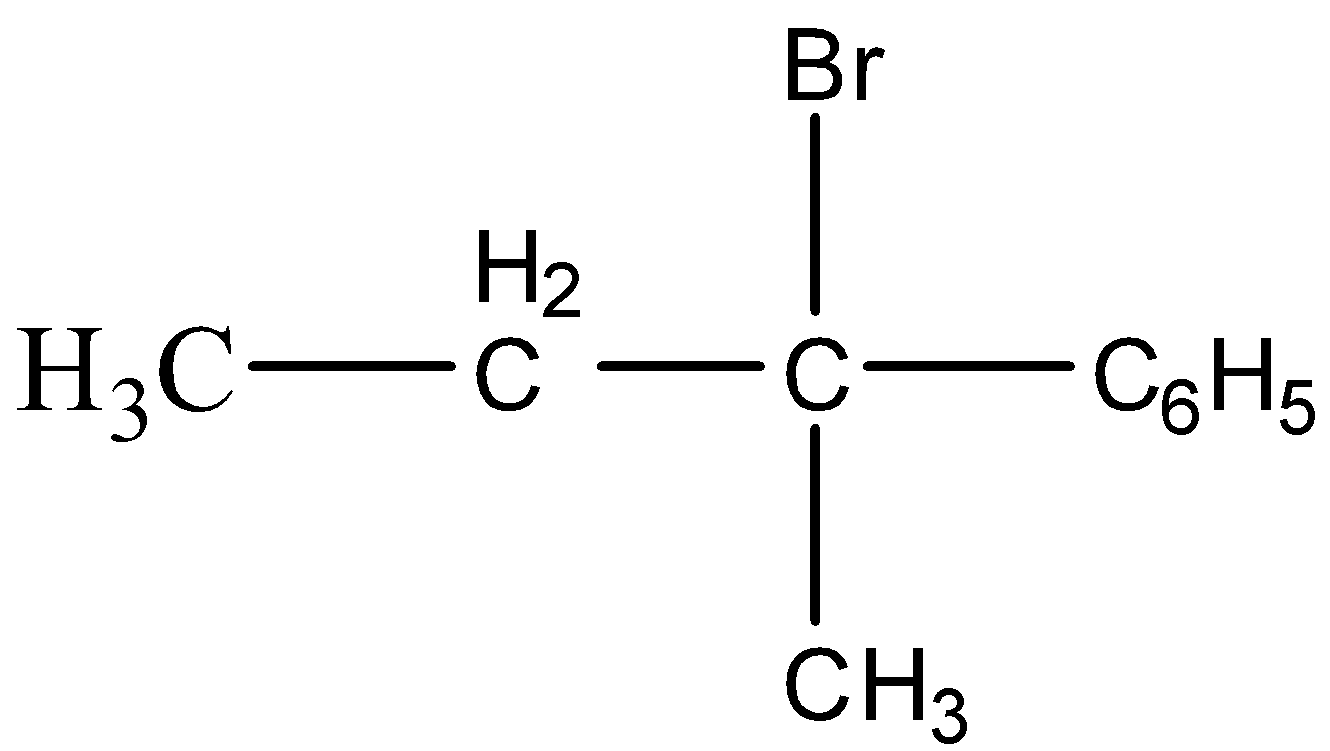
C.
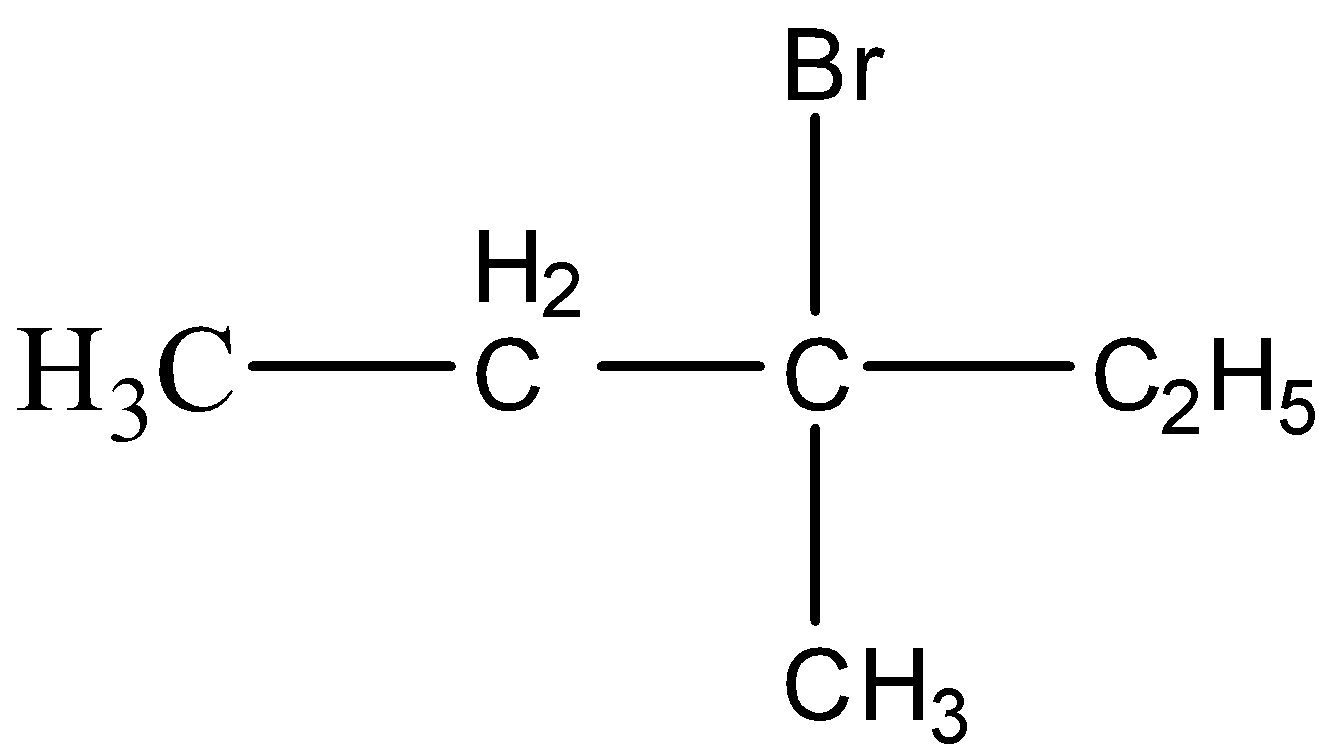
D.
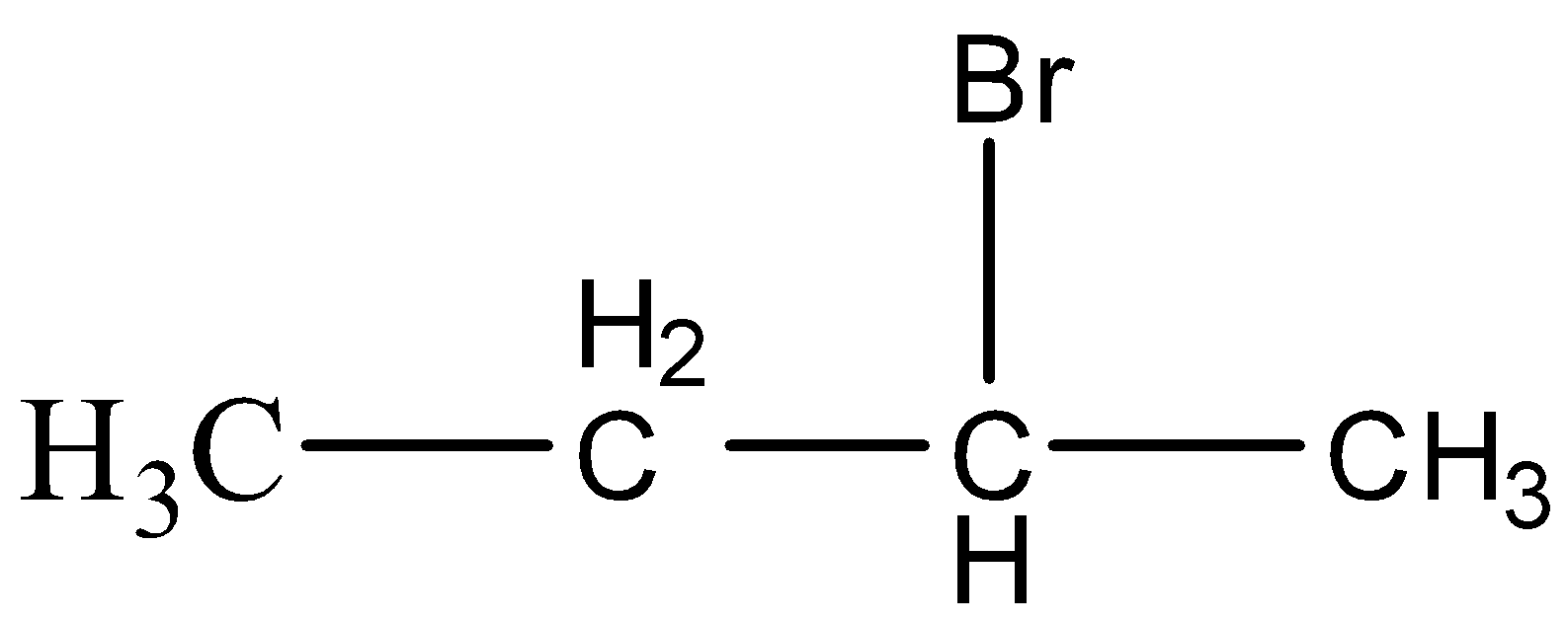
Solution
Due to elimination alkene is formed. Alkanes are aliphatic organic compounds which have a minimum of 1 and maximum ‘n’ number of carbon atoms. Also, only sigma bonds are present in alkanes. This means that alkanes are formed only with single bonds, and not double or triple bonds.
Complete step by step answer:
In the case of an E1 elimination reaction, at first, carbocation is formed. By the elimination of the leaving group. Then in the next step alkene formation takes place. In this case, the more substituted alkene is formed as a major product.
Elimination reaction is a steric assistance reaction. It means with increasing steric crowding the elimination reaction tendency increases and vice-versa.
Now, for E1 elimination, with increasing the carbocation stability the reaction rate will be increased and vice-versa. Now, the order of stability of carbocation depends upon the presence of electron-donating groups, the higher the number of electrons donating groups higher will be the stability of the carbocation and vice-versa.
Among the given options the most stable carbocation formation takes place for,
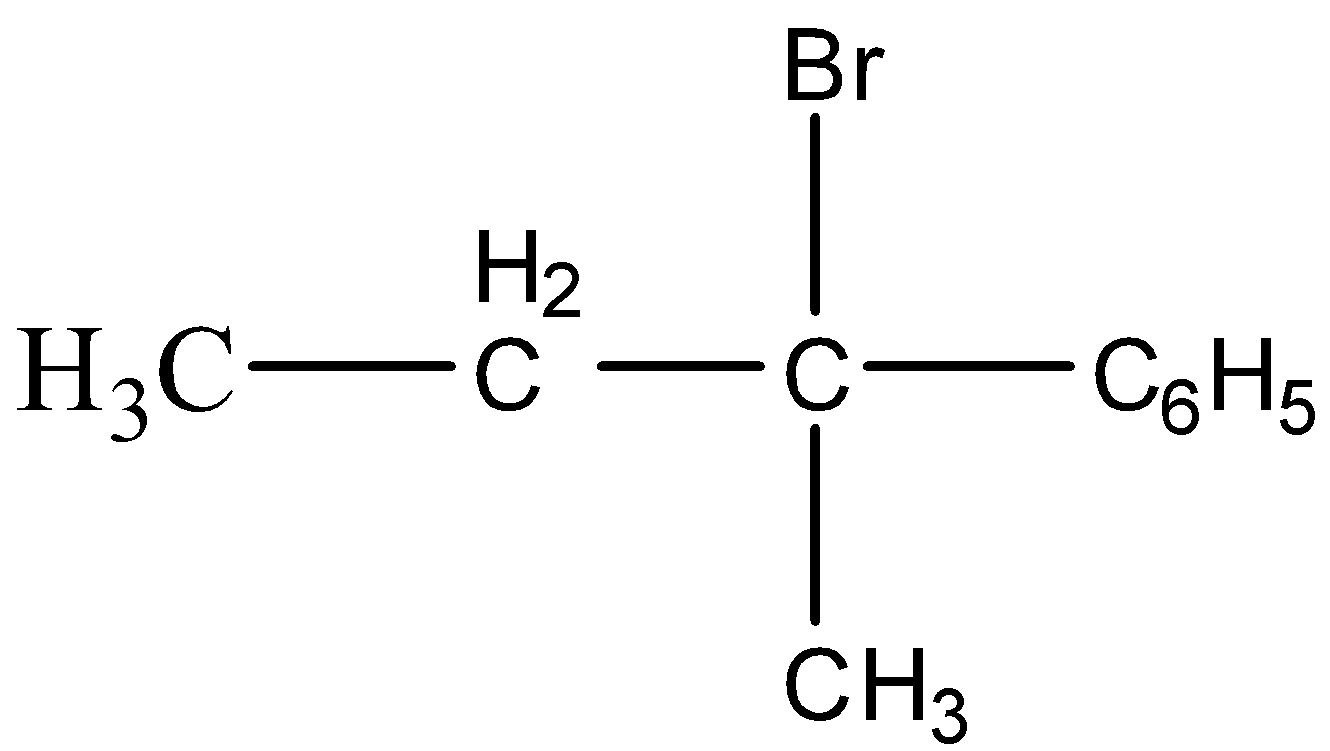
This is because the phenyl group donates the electrons by resonance. the reaction is shown below,
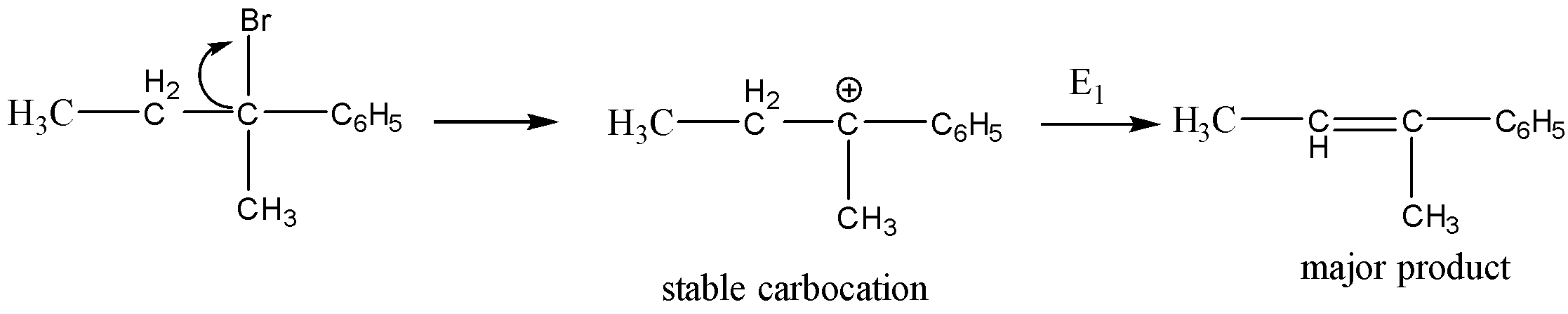
So, the correct option is B.
Note: Alcohol dehydration is an example of an elimination reaction in which a molecule is removed which leaves multiple bonds between the carbon atoms. In a dehydration reaction, the water molecule is lost from the compound called alcohol and results in an unsaturated compound like alkene.
