Question
Question: Which one of the following compounds gives carboxylic acid with\(HN{{O}_{2}}\)? A.
B.C6H5CONH2
C.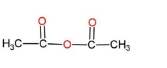
D.CH3COOC2H5
Solution
HNO2is known as nitrous acid. Nitrous acid reacts with an amide to yield carboxylic acid, while nitrogen gas is released. Amides can be hydrolyzed by acids as well as bases. Here acid is used to hydrolyze the compound.
Complete step-by-step answer: When nitrous acids react with amides then the products formed are carboxylic acids. This is also called hydrolysis of amides. The presence of amide linkage has the nitrous acid attack. The protonation occurs on the amide linkage, and then the water molecule attacks the carbonyl carbon atom, an intermediate is formed, which gives carboxylic acid. From the given compounds option B contains an amide linkage, which is benzamide, thus it will react with nitrous acid to yield carboxylic acid.
The reaction of amides with nitrous acid will give carboxylic acids, nitrogen gas, and water as the products, the reaction is as follows:
C6H5CONH2+HONO→C6H5COOH+N2+H2O
Hence, C6H5CONH2which is benzamide, is used to form carboxylic acid, benzoic acid with nitrous acid.
So, option B is correct.
Note: This reaction is of primary amide, the reaction of secondary amides with nitrous acids give N- nitroso chemical compounds, while tertiary amides do not react at all with nitrous acids. Amide hydrolysis is one of the important reactions in biology and is used to catalyze the peptides and proteins present in the body. This happens through proteolytic enzymes in the human body.
