Question
Question: Which one of the following complexes is an outer orbital complex? A. \({{[Fe{{(CN)}_{4}}]}^{6-}}\)...
Which one of the following complexes is an outer orbital complex?
A. [Fe(CN)4]6−
B. [Fe(CN)6]4−
C. [Co(NH3)6]3+
D. [Ni(NH3)6]2+
Solution
If a ligand is a strong ligand then the ligand forms an inner orbital complex with metals and if the ligand is weak then the ligand forms an outer orbital complex with metals. The hybridization of the inorganic complexes is going to depend on the type of ligand.
Complete step by step answer:
- To know about the structure of the complexes we should know about the hybridization involved in the metal complexes.
- Coming to given options, option A [Fe(CN)4]6− .
- The oxidation state of iron in [Fe(CN)4]6− is
x + 4(-1) = - 6
x = -2, here x= oxidation state of iron.
- The oxidation state of iron in [Fe(CN)4]6− is -2.
- The electronic configuration of iron is 1s22s22p63s23p64s23d6
- The hybridization of iron in [Fe(CN)4]6− is d2sp3 .
- Cyanide is a strong ligand then it forms an inner orbital complex with iron metal. So, option A is wrong.
- Coming to option B, [Fe(CN)6]4− .
- The oxidation state of iron in [Fe(CN)6]4− is
x + 6(-1) = - 4
x = 2, here x= oxidation state of iron.
- The electronic configuration of iron is 1s22s22p63s23p64s23d6
- The hybridization of iron in [Fe(CN)6]4− is d2sp3 .
- Cyanide is a strong ligand then it forms an inner orbital complex with iron metal. So, option B is wrong.
- Coming to option C, [Co(NH3)6]3+ .
- The oxidation state of cobalt in [Co(NH3)6]3+ is
x + 6(0) = 3
x = 3, here x= oxidation state of cobalt.
- The electronic configuration of Cobalt (3+) is 1s22s22p63s23p64s04p03d6
- Ammonia is a strong ligand and it forms an inner orbital complex with d2sp3 hybridization.
- So, option C is wrong.
- Coming to option D, [Ni(NH3)6]2+ .
- The oxidation state of nickel in [Ni(NH3)6]2+ is
x + 6(0) = 2
x = 2, here x= oxidation state of nickel.
- The electronic configuration of nickel (2+) is 1s22s22p63s23p63d84s04p04d0 .
- Ammonia is a strong ligand but due to the lack of 3d orbitals of nickel participation in the hybridization, ammonia forms an outer orbital complex with nickel.
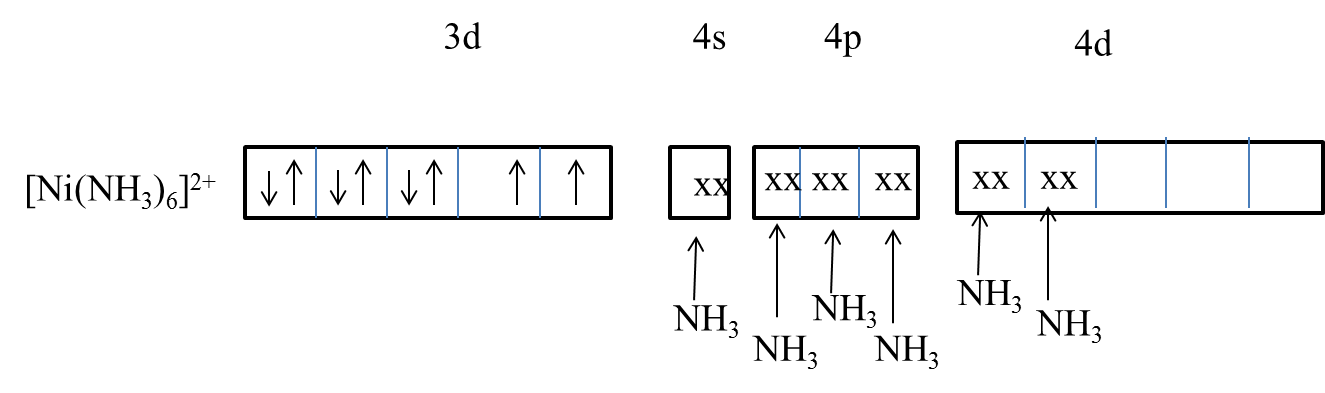
- Ammonia forms an outer orbital complex with sp3d2 hybridization with nickel.
- So, the correct option is D.
Note: An inorganic metal complex in which the central metal atom utilizes outer d orbitals for hybridization then the complex is called outer orbital complex. An inorganic metal complex in which the central metal atom utilizes inner d orbitals for hybridization then the complex is called inner orbital complex.
