Question
Question: Which one of the following carbocations is most stable? A. 
B.
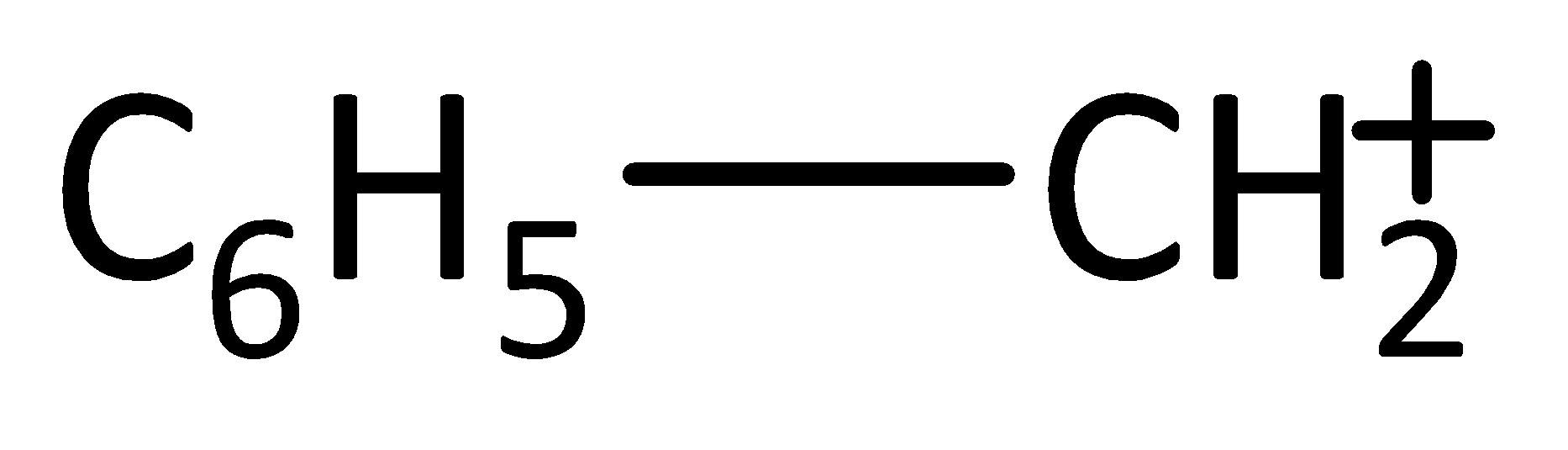
C.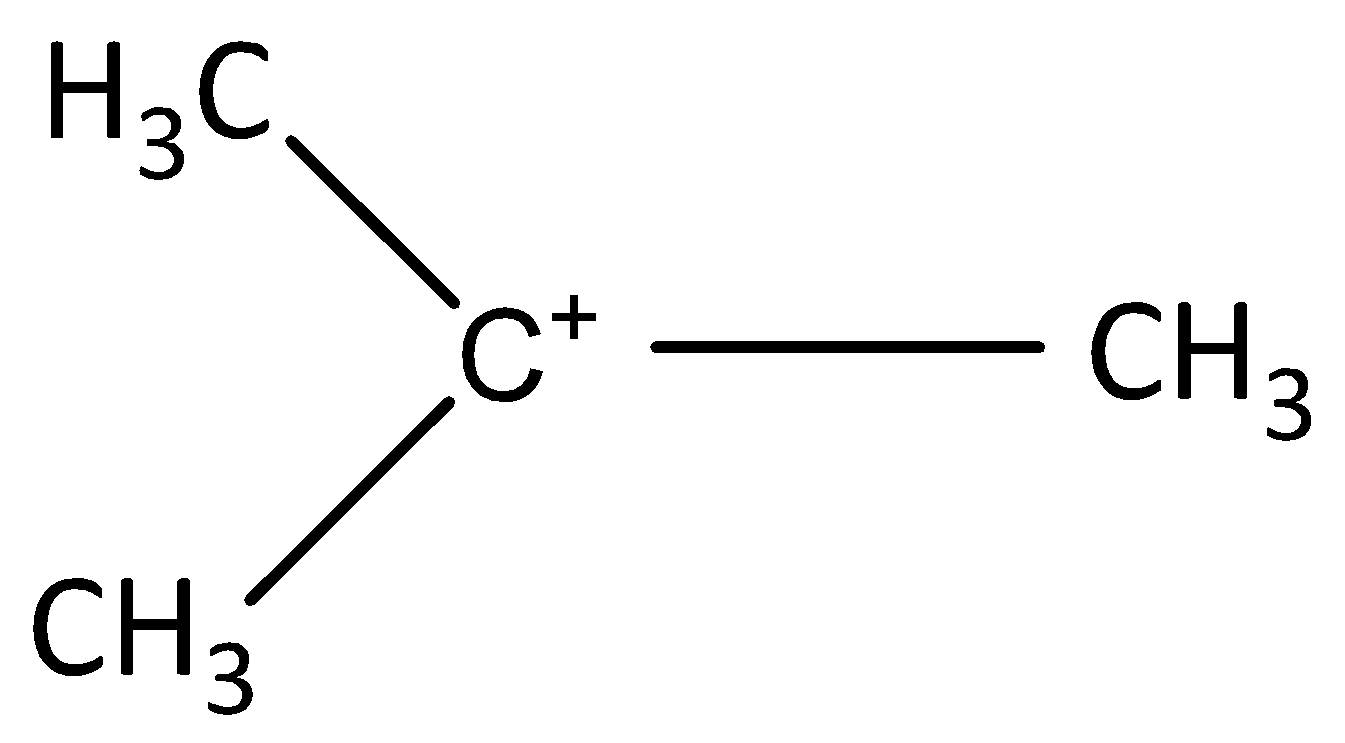
D.
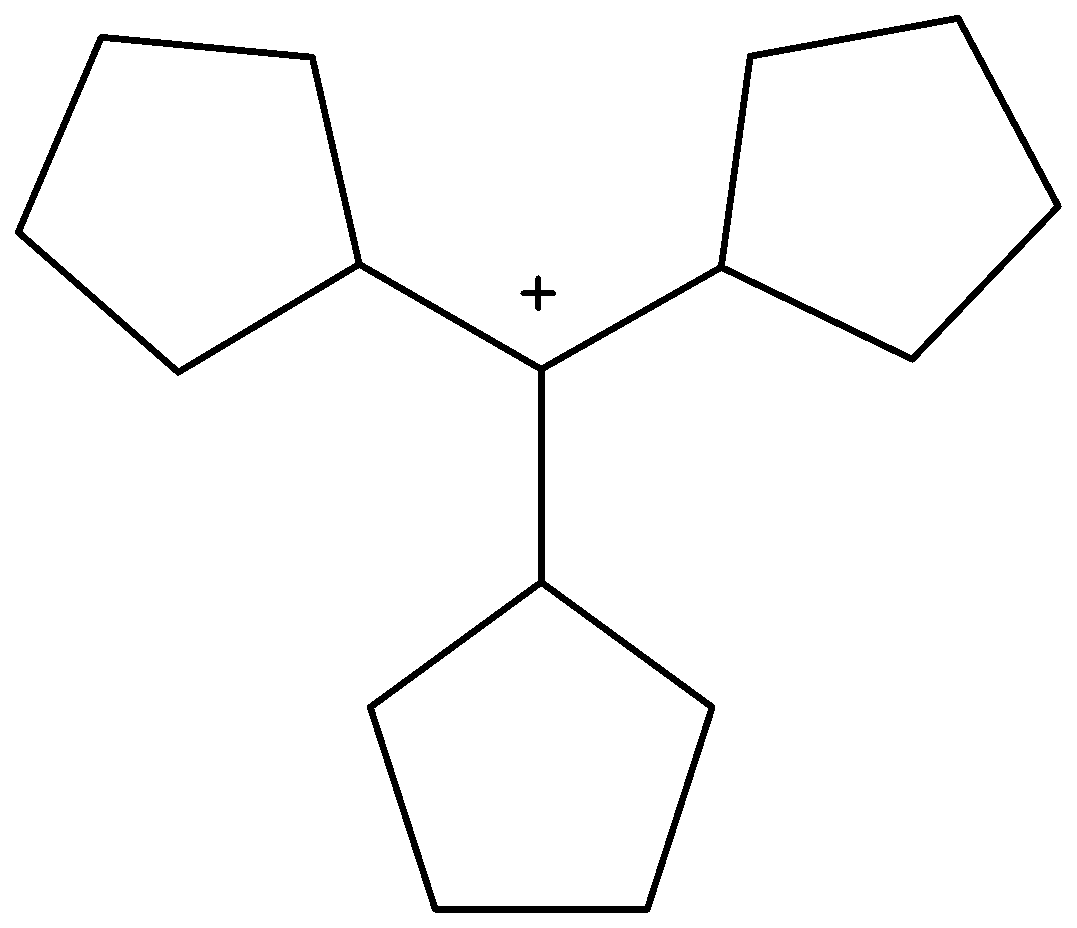
Solution
We need to know what carbocations are and what influences their stability. A carbon atom basically can bond to 4 other atoms. A carbocation is formed when a carbon atom has one positive charge and three other bonds. It is also sometimes referred to as carbonium ion. Since they do not satisfy the octet rule, they are very unstable and reactive and hence the nature of instability is a topic to be discussed about.
Complete step by step answer:
A variety of factors influence the stability of carbocations. They are discussed below:
-Resonance: Resonance describes the delocalization of electrons in a molecule in a number of resonance structures based on the possibilities of electron delocalization. In case of carbocations, there is delocalization of the positive charge and more the number of resonating structures, the more stable is the carbocation.
-Electronegativity: The potential of an atom to attract electrons is called its electronegativity. Electrons being negatively charged and therefore the carbon of the carbocation with positive charge influences the stability of the carbocation. The stability of the carbocation decreases with increase in electronegativity. Electronegativity in turn depends on hybridization of the carbocation. The sp type of hybridization has the maximum s character and thus maximum electronegativity.
-Hyperconjugation: The stability of tertiary carbocations is more due to hyperconjugation. In other words, it can be said that the stability of carbocations depends on the number of groups attached directly to the carbon carrying the positive charge.
One major exception in the stability of carbocations can be explained by the carbocation (A) which has 3 cyclopropane rings attached to the carbon atom carrying the positive charge. A fragment of cyclopropane behaves like a double bond and therefore has a dancing resonance and therefore tri cyclopropane carbocation is the most stable carbocation.
Hence without analyzing the other carbocations, it can be said that option (A) is the most stable carbocation.
-In addition, the stability of (B) is due to resonance only. The stability of (C) is described with the help of hyperconjugation and inductive effect. On the basis of hyperconjugation, it shows six resonating structures due the presence of six C-H bonds. The carbocation (D) has three cyclopentane rings for delocalization hence stable.
Therefore, the option B, C and D are incorrect.
Therefore, the correct option is option (A).
Note:
We must be noted that the most stable carbocation is tropylium cation where the positive charge is distributed over 7 carbon atoms. The stability of cyclopropyl groups is far below tropylium cation. The bonds in cyclopropane are called bent bonds and the resonance is called bent bond resonance and stabilizes the positive charge better than a phenyl group.
