Question
Question: Which one of the following alkenes will react faster with \(H_2\) under catalytic hydrogenation cond...
Which one of the following alkenes will react faster with H2 under catalytic hydrogenation conditions?
A. 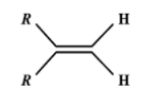
B. 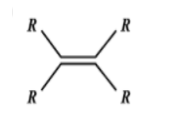
C.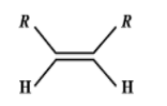
D. 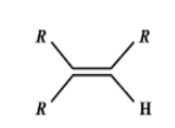
Solution
Hint : Students must know that stability of alkene is inversely proportional to the heat of hydrogenation of alkene. Therefore as the hydrogenation increases/decreases the stability of alkene will increase or decrease depending upon the process of hydrogenation.
Complete step by step answer:-
Hydrogenation is a chemical process in which molecular hydrogen reacts with compounds or elements present in the presence of catalysts such as platinum, nickel etc. During this process the hydrogen attaches with the molecule having a double or triple bond. Resulting in dissociation of molecules. During catalytic hydrogenation, The hydrogen is transferred from the catalyst to the same side of the double bond.
Evidently, small the number of R-substituents, lesser is the steric hindrance and hence faster is the rate of hydrogenation..
Catalytic hydrogenation takes place in the 3rd compound. The energy required to break the double bond is lesser in the 3rd compound than any other compound. Greater the number of alkyl groups attached to the doubly bonded carbon atoms, more stable is the alkene. (less reactive).
Thus the option (a) with two groups on the same side of the molecule is correct.
**Hence (A) is the correct answer
Note : **
A student may forget the inverse relation of heat of hydrogenation and stability. One may also get perplexed between the converse effect of R-substituents on the rate of hydrogenation. It is advised to take proper notes on the effect of stability on alkenes.
