Question
Question: Which one of the following alcohols are oxidised by $MnO_2$?...
Which one of the following alcohols are oxidised by MnO2?
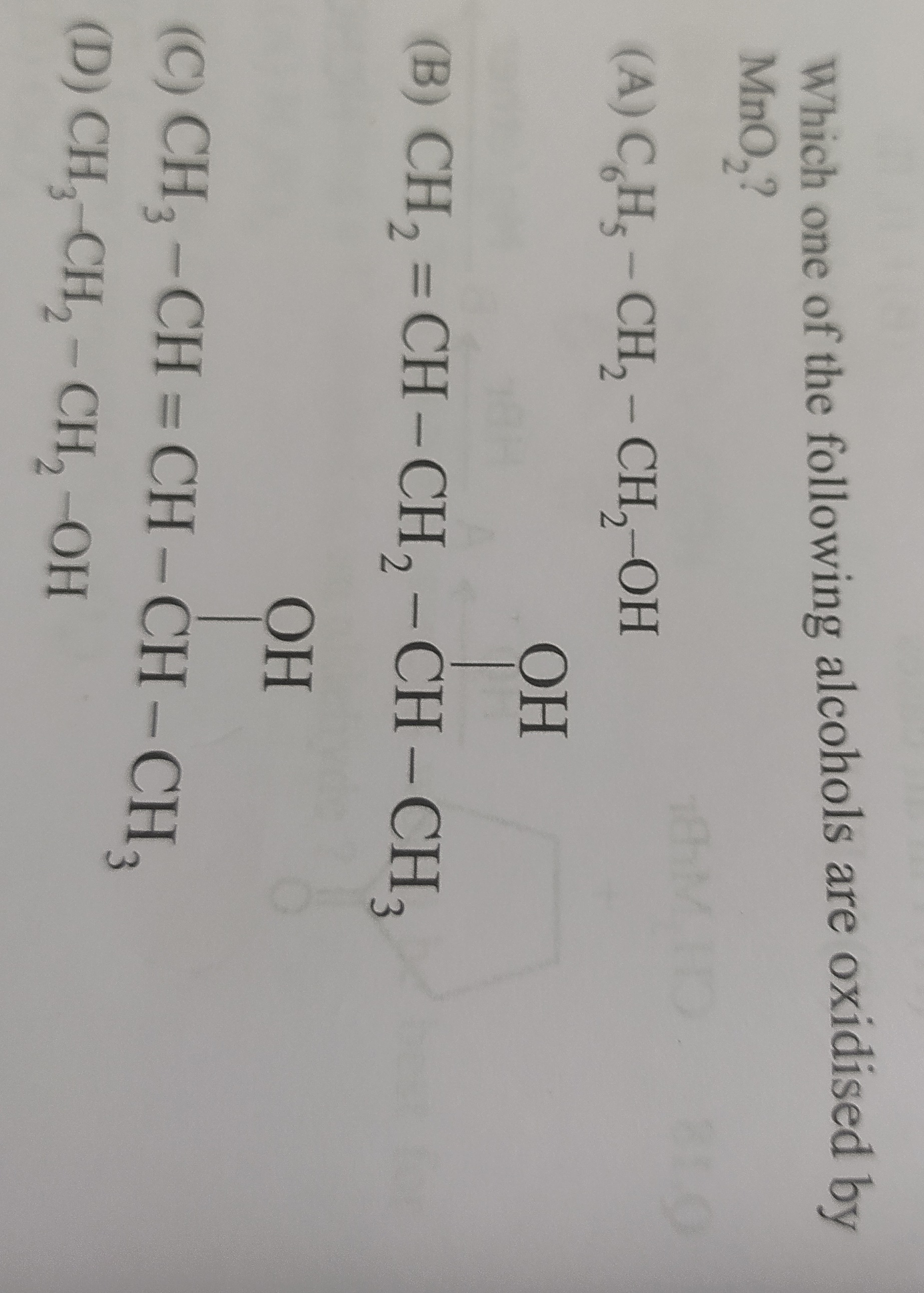
A
C6H5−CH2−CH2−OH
B
CH2=CH−CH2−CH(OH)−CH3
C
CH3−CH=CH−CH(OH)−CH3
D
CH3−CH2−CH2−OH
Answer
The correct options are (B) and (C).
Explanation
Solution
MnO2 is a mild oxidizing agent that selectively oxidizes allylic and benzylic alcohols to carbonyl compounds (aldehydes or ketones). It does not oxidize saturated primary or secondary alcohols.
- (A) C6H5−CH2−CH2−OH: This is 2-phenylethanol. The hydroxyl group is on a primary carbon, but this carbon is not directly attached to the phenyl ring. Therefore, it is not a benzylic alcohol. MnO2 does not oxidize this compound.
- (B) CH2=CH−CH2−CH(OH)−CH3: This molecule contains a hydroxyl group on a secondary carbon that is adjacent to a CH2 group, which is part of an alkene (CH2=CH−). Thus, it is a secondary allylic alcohol. MnO2 oxidizes allylic alcohols to ketones.
- (C) CH3−CH=CH−CH(OH)−CH3: This molecule contains a hydroxyl group on a secondary carbon that is adjacent to a CH group, which is part of an alkene (CH3−CH=CH−). Thus, it is a secondary allylic alcohol. MnO2 oxidizes allylic alcohols to ketones.
- (D) CH3−CH2−CH2−OH: This is propan-1-ol, a saturated primary alcohol. MnO2 does not oxidize saturated alcohols.
