Question
Question: Which one of the following acids does not exhibit optical isomerism? (A) Lactic acid (B) Tartari...
Which one of the following acids does not exhibit optical isomerism?
(A) Lactic acid
(B) Tartaric acid
(C) Maleic acid
(D) α−amino acids
Solution
For determining which of the acids does not exhibit optical isomerism, we know that only those compounds exhibit optical isomerism, which has a chiral centre or absence of symmetry elements.
Complete step by step solution:
We have been provided with acids: Lactic acid, Tartaric acid, Maleic acid and α−amino acids,
We need to tell which one of them would exhibit optical isomerism,
So, for that:
First acid we have lactic acid:
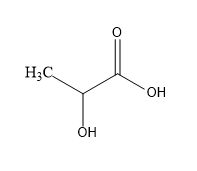
So, in lactic acid one chiral centre is present so it is optically active.
Next, we have Tartaric acid:
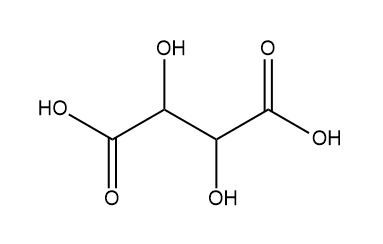
So, in tartaric acid two chiral centres are present so it is optically active.
Next, we have Maleic acid:
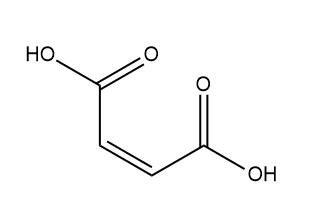
So, Maleic acid does not have any chiral centre, so it does not exhibit optical isomerism.
Last one we have α−amino acid:
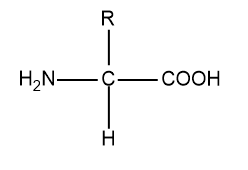
So, in α−amino acid one chiral centre is present so it is optically active.
So, we can say that Maleic acid would not exhibit optical isomerism.
Therefore, we can conclude that option (C) is correct.
Note: Optical isomers are important because two isomers can have the same chemical formula, but have different chemical structures. And these structures contribute to the properties of the molecule.
