Question
Question: Which one of following has least magnetic moment...
Which one of following has least magnetic moment
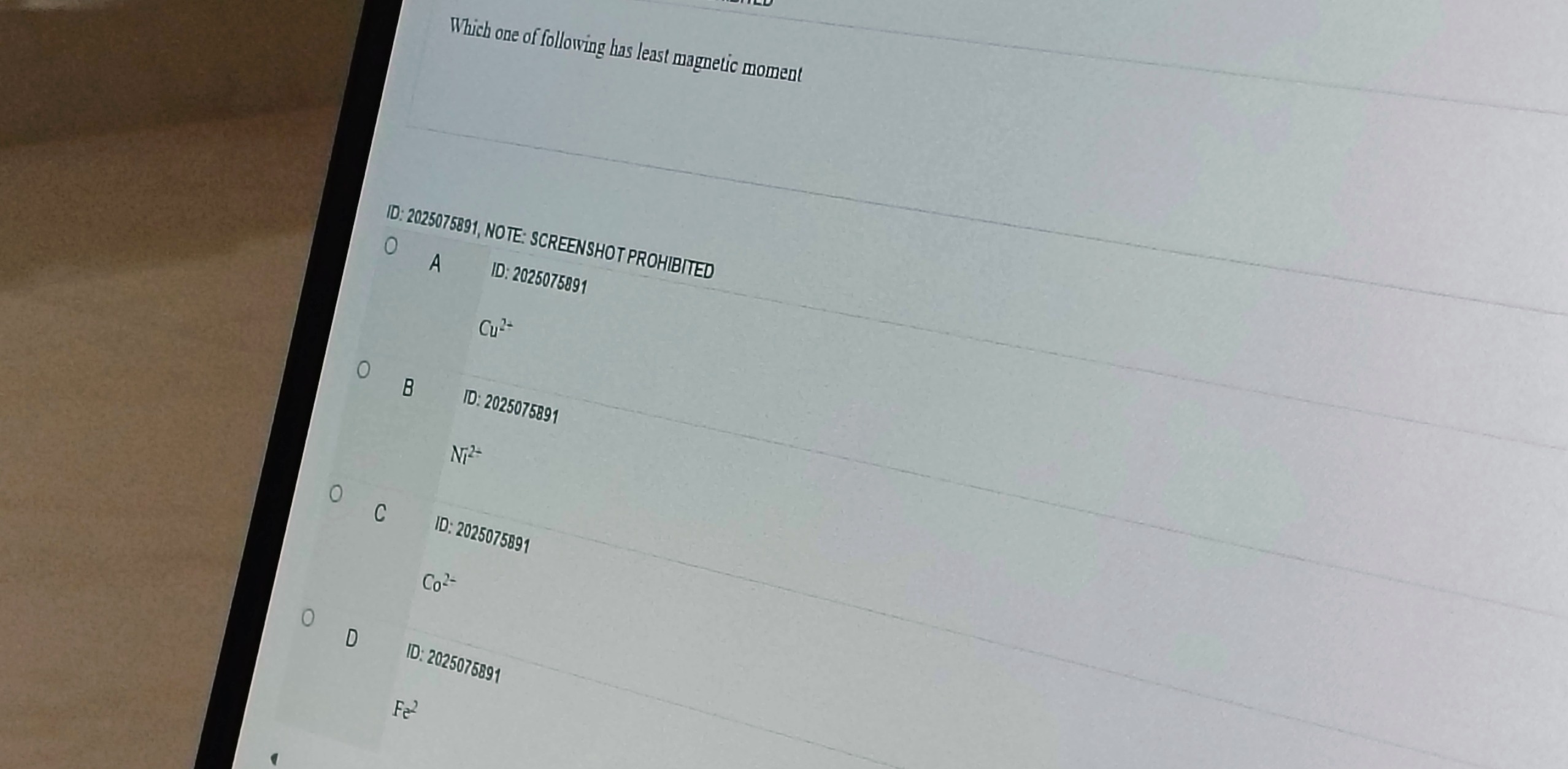
Cu2+
Ni2+
Co2−
Fe2
A
Solution
To determine which ion has the least magnetic moment, we need to find the number of unpaired electrons (n) for each ion. The magnetic moment (μ) is given by the spin-only formula:
μ=n(n+2) BM (Bohr Magnetons)
A smaller number of unpaired electrons (n) results in a smaller magnetic moment.
Let's analyze each option, assuming the common stable oxidation states for transition metals and interpreting the given options as such (e.g., Co2− as Co2+ and Fe2 as Fe2+).
-
Cu2+
- Atomic number of Cu = 29
- Electronic configuration of Cu: [Ar]3d104s1
- Electronic configuration of Cu2+: [Ar]3d9 (one electron removed from 4s, one from 3d)
- For 3d9, there is 1 unpaired electron. d-orbitals: ↑↓↑↓↑↓↑↓↑
- Number of unpaired electrons (n) = 1
- Magnetic moment μ=1(1+2)=3≈1.73 BM
-
Ni2+
- Atomic number of Ni = 28
- Electronic configuration of Ni: [Ar]3d84s2
- Electronic configuration of Ni2+: [Ar]3d8 (two electrons removed from 4s)
- For 3d8, there are 2 unpaired electrons. d-orbitals: ↑↓↑↓↑↓↑↑
- Number of unpaired electrons (n) = 2
- Magnetic moment μ=2(2+2)=8≈2.83 BM
-
Co2+ (assuming Co2− is a typo for Co2+)
- Atomic number of Co = 27
- Electronic configuration of Co: [Ar]3d74s2
- Electronic configuration of Co2+: [Ar]3d7 (two electrons removed from 4s)
- For 3d7, there are 3 unpaired electrons (in high spin configuration, which is typical for free ions). d-orbitals: ↑↓↑↓↑↑↑
- Number of unpaired electrons (n) = 3
- Magnetic moment μ=3(3+2)=15≈3.87 BM
-
Fe2+ (assuming Fe2 is a typo for Fe2+)
- Atomic number of Fe = 26
- Electronic configuration of Fe: [Ar]3d64s2
- Electronic configuration of Fe2+: [Ar]3d6 (two electrons removed from 4s)
- For 3d6, there are 4 unpaired electrons (in high spin configuration). d-orbitals: ↑↓↑↑↑↑
- Number of unpaired electrons (n) = 4
- Magnetic moment μ=4(4+2)=24≈4.90 BM
Comparing the number of unpaired electrons:
- Cu2+: n = 1
- Ni2+: n = 2
- Co2+: n = 3
- Fe2+: n = 4
The ion with the least number of unpaired electrons is Cu2+ (n=1). Therefore, Cu2+ has the least magnetic moment.
