Question
Question: Which one is the most acidic compound? 
Solution
Acidity can be referred to as "electron pair instability", and this electron pair instability increases as the charge density on the phenol decreases. This means that the acidity should or does
increase with the increased positive charge on the group. And this increased charge on the group is due to the presence of the electron withdrawing group attached to the main group.
Complete step by step answer:
Acidity of a compound can be defined as its ability to donate a pair of electrons.
To determine the acidity of a compound, we need to see what type of substituent group is attached to the compound (phenol is this case).
A. If the substituent group attached is an electron releasing group (ERG), then they decrease the acidity of the compound due to their +I and +R effect. The more the number of ERG attached, the less will be the acidity of the compound. For example, −CH3 group is an ERG due to its +I effect.
B. If the substituent group attached is an electron withdrawing group (EWG), then they increase the acidity of the compound due to their -I and -R effect. The more the number of EWG attached, the more will be the acidity of the compound. For example, −NO2 group is an EWG due to its -R effect.
Now let us look at the options given to us one by one.
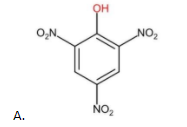
The above compound contains three EWG (−NO2) as substituent groups. Therefore, it is the most acidic compound.
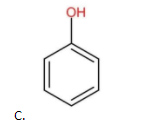
The above compound does not contain any −NO2 as the substituent group. Therefore, it is less acidic than (A) but more acidic than (B).
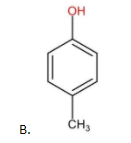
The above compound does not contain any −NO2 as the substituent group. Therefore, it is less acidic than (A) but more acidic than (B).
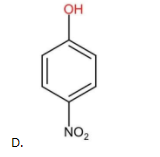
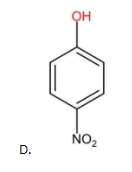
The above compound contains only one EWG (−NO2) as the substituent group. Therefore, it is less acidic than (A) but more acidic than (B) and (C).
Therefore, the decreasing order of the acidity of the compound is A > D > C > B.
Hence, the correct option is option (A).
Note: If the one nitro group in option D that is present in the para position was present in the meta position instead then also the p-nitrophenol is more acidic than m-nitrophenol. m-nitrophenol is also less acidic than o-nitrophenol in which the nitro group is present in the ortho position. But o-nitrophenol is less acidic than p-nitrophenol. Therefore the acidity order is p-nitrophenol> o-nitrophenol> m-nitrophenol.
