Question
Question: Which one is classified as a condensation polymer? (A). Teflon (B). Acrylonitrile (C). Dacro...
Which one is classified as a condensation polymer?
(A). Teflon
(B). Acrylonitrile
(C). Dacron
(D). Neoprene
Solution
Polymers are the long chain molecules that are made up of small repeating units. These small repeating units are known as monomers. They can occur naturally or can be chemically synthesized. The process of formation of polymers from monomers, is known as polymerization.
Complete step by step answer:
Condensation polymers are those polymers that are formed from monomers by losing small molecules as byproducts each time a bond is formed between the two monomers. These small molecules which are formed as a byproduct are after water (H2O) or carbon dioxide(CO2).
(A).Teflon is also known as polytetrafluoroethylene. It is polymerised from the monomer, called tetrafluoroethylene. Its reaction of polymerization can be written as:
nCF2=CF2→-(F2C−CF2-)n
(tetrafluoroethylene) (Polytetrafluoroethylene)
Since, no byproduct is formed in this polymerization reaction, thus Teflon is not a condensation polymer.
(B).Acrylonitrile is a monomer which is used to polymerise polyacrylonitrile. This reaction can be written as:
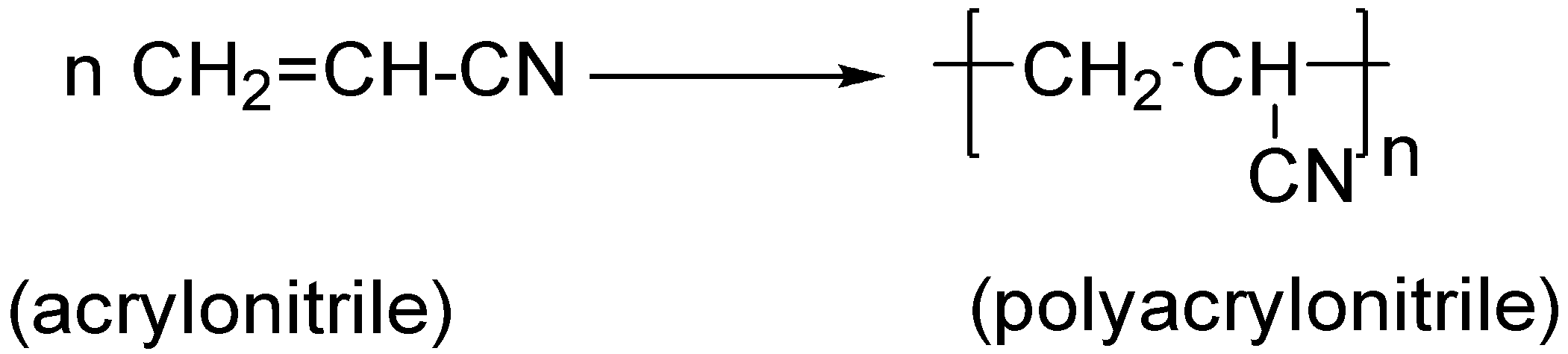
Thus, this is also not a condensation polymer in fact acrylonitrile is itself a monomer.
(C).Dacron is also known as terylene. It is polymerized from two monomers that are ethylene glycol and terephthalic acid. Its polymerization reaction can be written as:
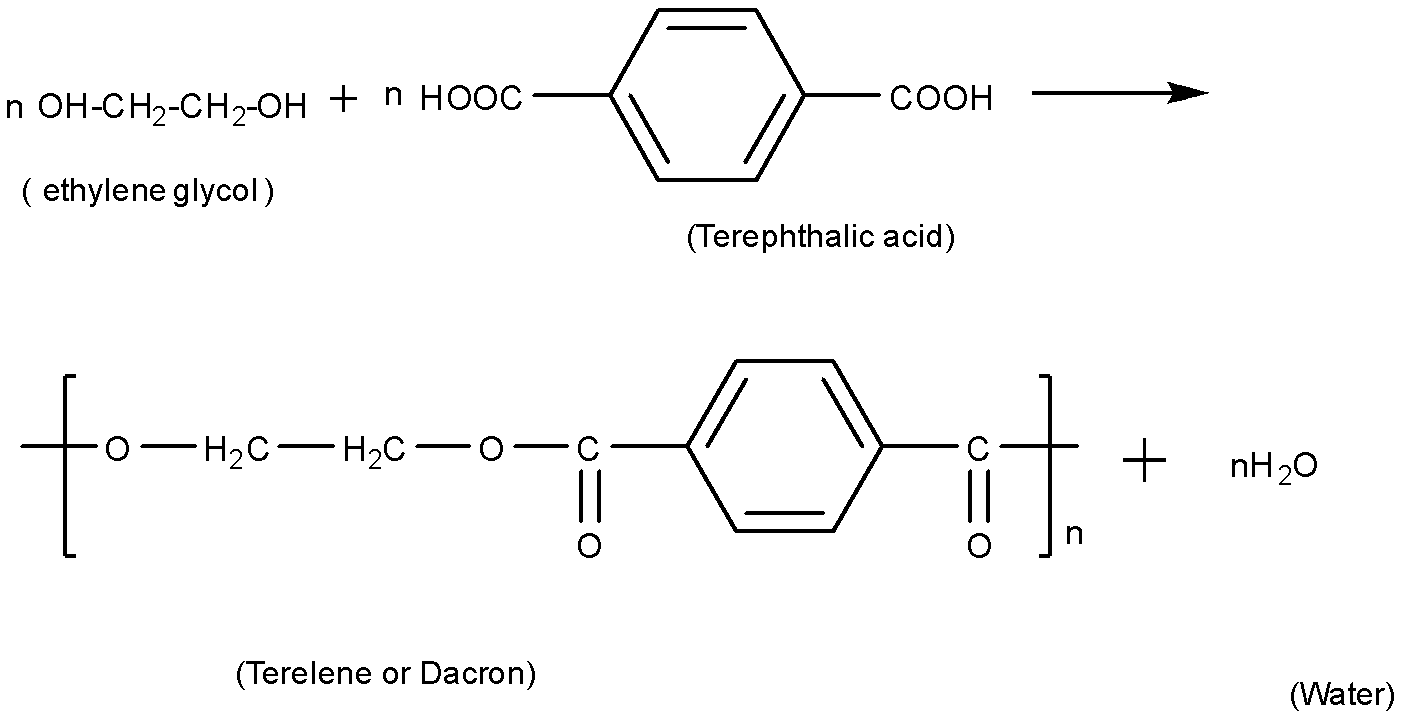
In this polymerisation reaction, water molecules are formed as a by-product, thus this is a condensation polymer.
(D). Neoprene is polymerised from its monomer called isoprene and the reaction can be written as:
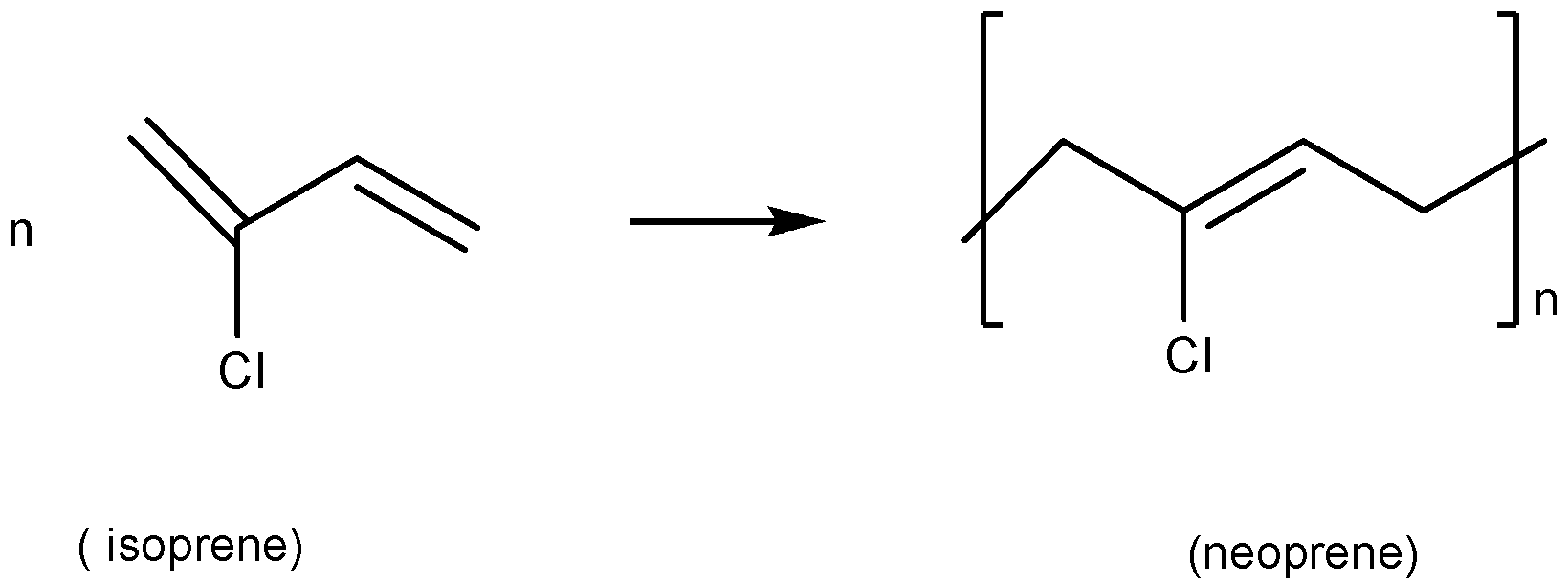
Thus it is also not a condensation polymer.
So, the correct answer is Option C.
Note: As opposed to condensation polymers, the polymers which are polymerised without forming any by-product are known as the addition polymers. The condensation polymers form more slowly than the additional polymers and often require heat.
