Question
Question: Which one among the following cannot exhibit enantiomerism? A. Diphenyl methanol B. \({\rm{1 - B...
Which one among the following cannot exhibit enantiomerism?
A. Diphenyl methanol
B. 1−Bromo−2−chlorobutane
C. 2−Butanol
D. Tartaric acid
E. None of the above
Solution
We know that the enantiomers are those compounds which give the mirror image of the compound and the isomerism of the enantiomers compound is termed as enantiomerism. Generally, they are non-superimposable images.
Complete step by step solution
As we all know, the enantiomers are not identical and they show chiral effects. The compounds which have no chiral carbon that compound does not show enantiomerism property. All the compounds which are given in the question are Diphenyl methanol, 1−Bromo−2−chlorobutane, 2−Butanol, and Tartaric acid. The enantiomers are unlike only in their optical activity that generally means the direction in which the enantiomer rotates plane polarized light. The chiral center is that in which the carbon atom is bonded with four different chemical species. The structure which exhibits the chiral centers are 1−Bromo−2−chlorobutane, 2−Butanol, and Tartaric acid is shown below.
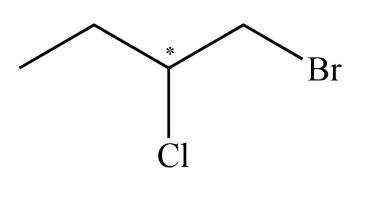
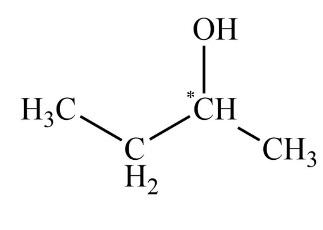
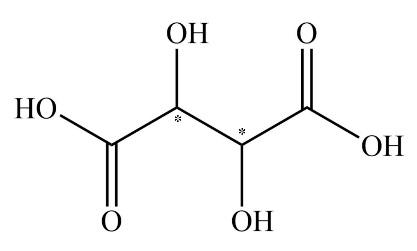
The compound diphenyl methanol does not have any chiral carbon. So, according to the principle of enantiomerism, if compounds do not have chiral carbon then they are not enantiomers and they do not show enantiomerism. The diagram of diphenyl methanol is shown below.
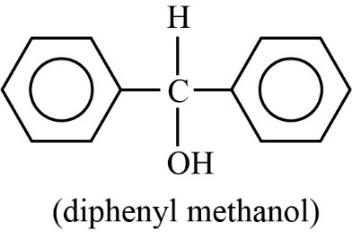
Thus, diphenyl methanol cannot exhibit enantiomerism.
**Hence, the correct option for this question is A that is diphenyl methanol.
Note: **
When the enantiomer is in excess this state is termed as enantiomeric excess. The enantiomeric excess generally can be measured by the purity used for that chiral substance. It also reflects the degree to which the given compound contains one enantiomer in bigger amounts than the other enantiomer compound.
