Question
Question: Which of unsaturated aldehydes (a)-(d) is the sigmatropic rearrangement product obtained by heating ...
Which of unsaturated aldehydes (a)-(d) is the sigmatropic rearrangement product obtained by heating the following ether?
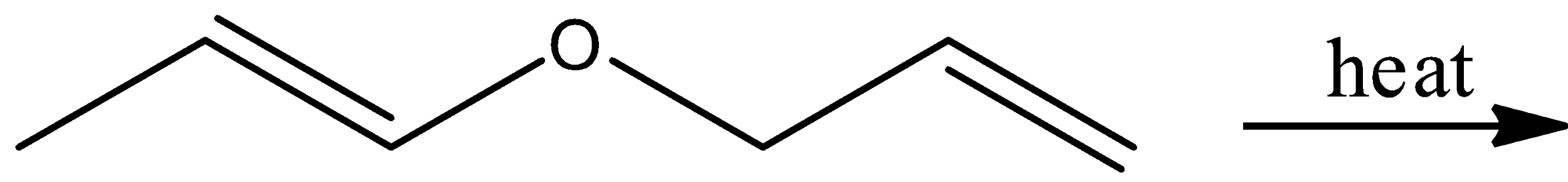
A.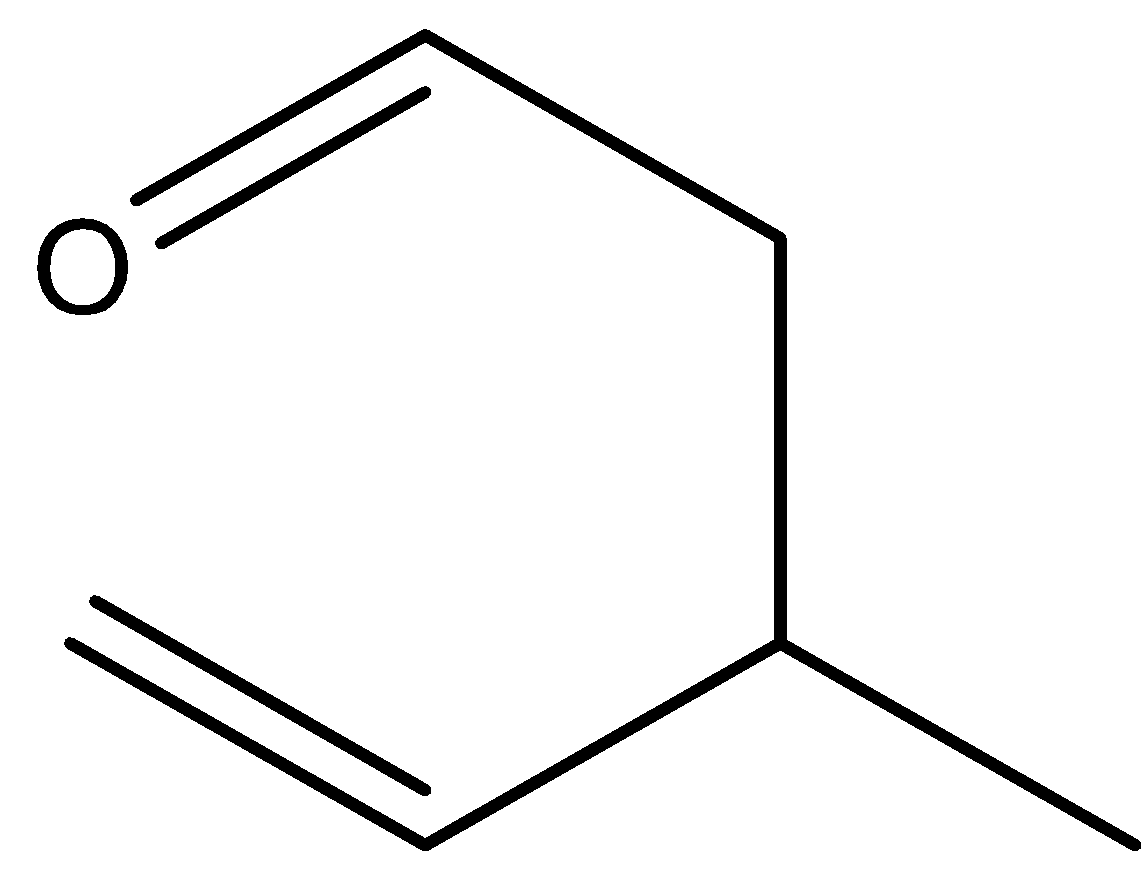
B.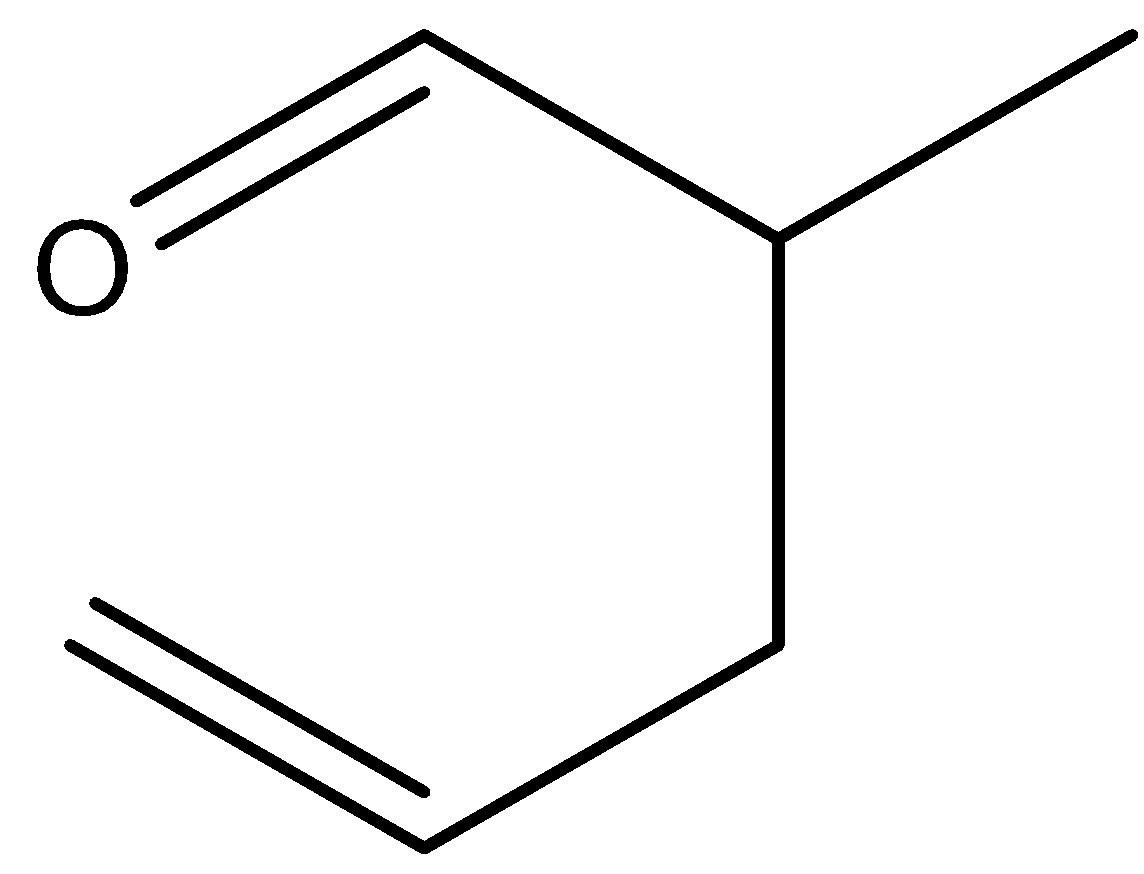
C.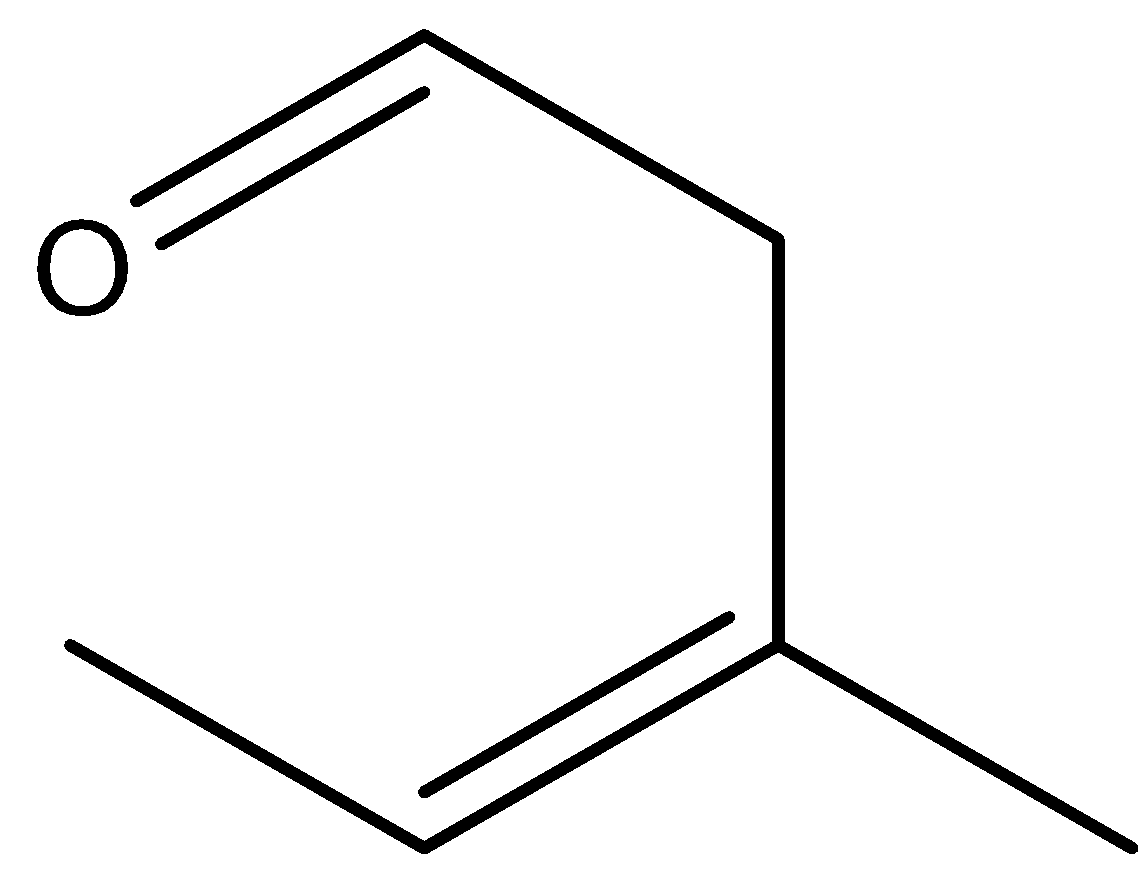
D.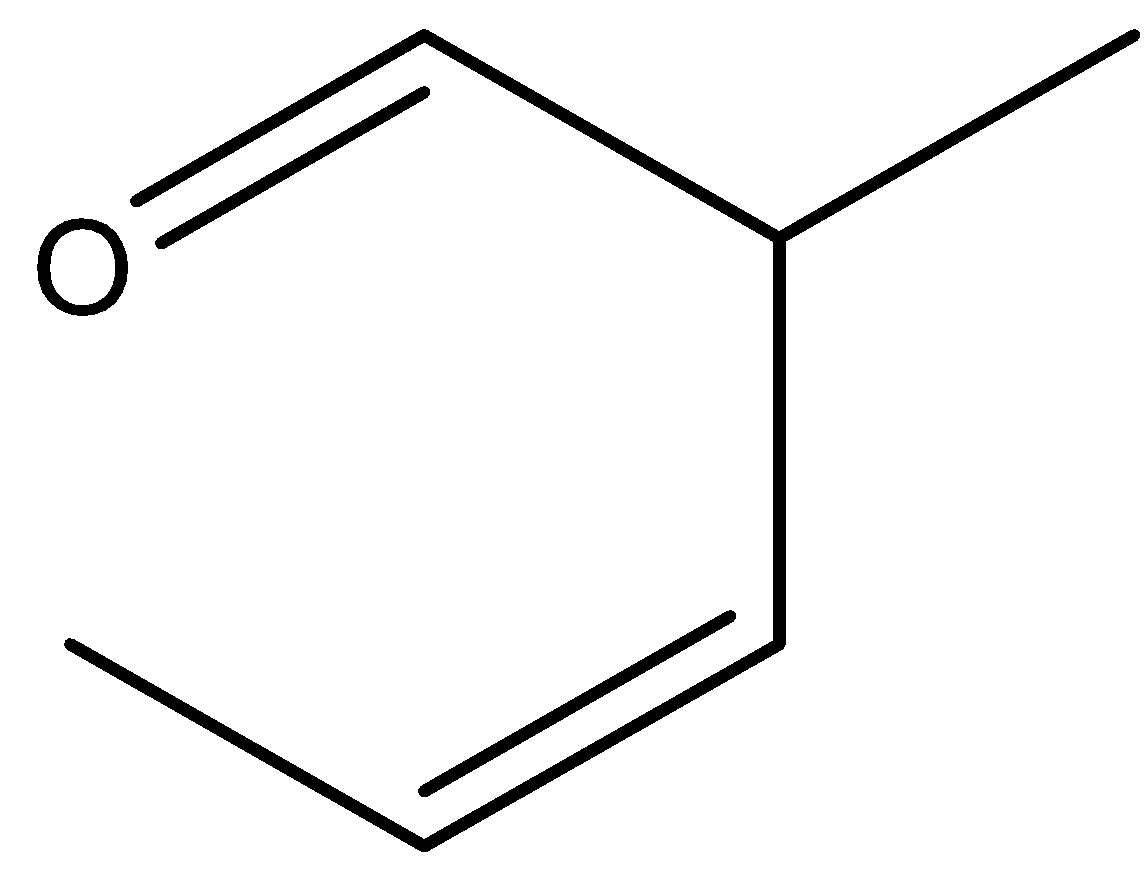
Solution
We must have to know that the aldehyde is a chemical compound having the formula R−CHO and it has C=O functional group. Here, the unsaturated carbon – carbon bond is present in the alpha and beta position and they are conjugated with a double bond. We must have to know that the alpha- beta unsaturated carbonyl compounds are allowed to attack nucleophiles at the beta position of the carbonyl group.
Complete answer:
By the heating of (E)-1-(allyloxy) prop-1-ene there is not a formation of 3-methylpent-4-enal. Hence, option (A) is incorrect.
When (E)-1-(allyloxy) prop-1-ene is reacted under heat, there will not be 2-methylpent-4-enal. Hence, the option (B) is incorrect.
Here, the reactant is an unsaturated aldehyde and its name is(E)-1-(allyloxy) prop-1-ene. By heating this aldehyde, it undergoes sigmatropic rearrangement and there is a formation of ether, which is (Z)-3-methylpent-3-enal. Let’s see the reaction,
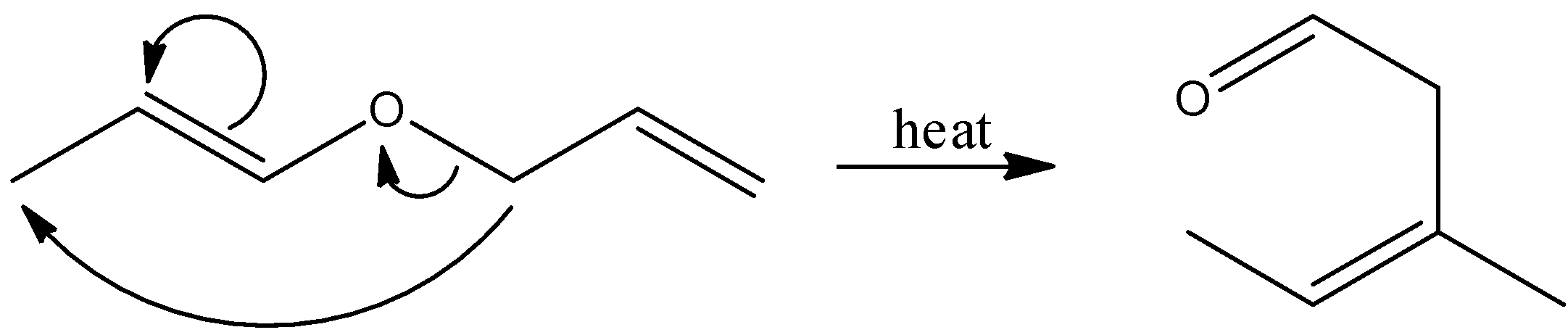
Hence, option (C) is correct.
By the sigmatropic rearrangement of unsaturated aldehydes, there will not be a formation of (Z)-2-methylpent-3-enal. Hence, the option (D) is incorrect.
Note:
We must have to remember that the rearrangement reactions are classified in many types, sigma tropic rearrangement, claisen rearrangement etc. during the rearrangement reaction, the organic molecule is rearranged and there is a formation of structural isomer. For example, the isomerization of n- butane gives isobutene. And in the case of sigmatropic rearrangement, there is a migration of a sigma bond which is adjacent to the one or more pi bond. Hydrogen and its isotope are common examples which undergo the sigmatropic shift.
