Question
Question: Which of these undergo polymerization? A.\[C{H_3}OH\] B.\[{C_2}{H_5}OH\] C.\[C{H_3} - CO - C{H...
Which of these undergo polymerization?
A.CH3OH
B.C2H5OH
C.CH3−CO−CH3
D.CH3CHO
Solution
In the case of polymerization is a chemical reaction, in that the monomer is reacting jointly and there is a formation of 3D-networks or a polymer chain. And the monomers are very small molecules and after polymerization reaction, there is a formation of large size molecules which is known as polymer. The monomer may be the mixture of two or more different compounds.
Complete answer:
Methanol is a simple alcohol having the formula, CH3OH. And it does not undergo the polymerization reaction. Whereas, methanol is used to prevent the polymerization reaction. Hence, option (A) is incorrect.
Ethanol is a chemical compound with a chemical formula, C2H5OH. And it does not undergo the polymerization reaction. Hence, the option (B) is incorrect.
The acetone is a chemical compound having the molecular formula, CH3CHO−CH3. And it undergoes the polymerization reaction. Here, one or more monomer units combined together to form a polymer and the structure is,
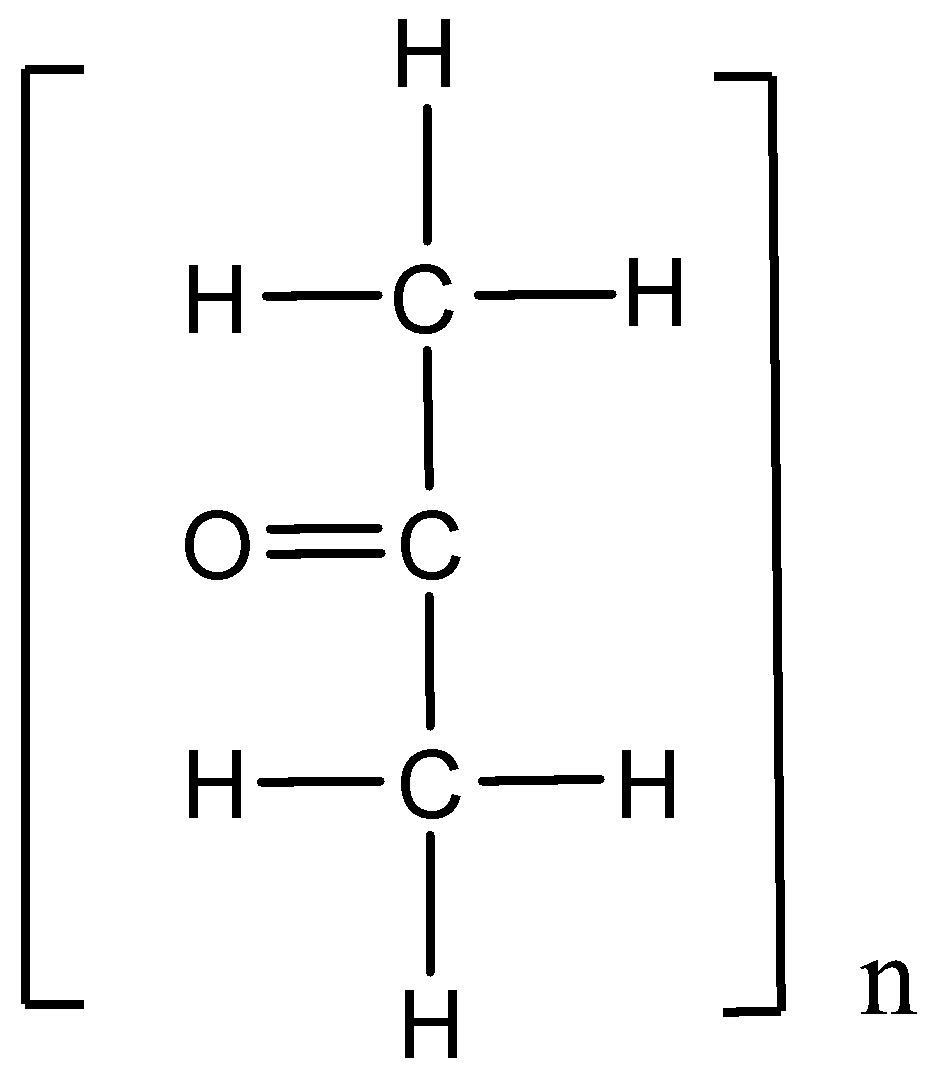
Hence, option (C) is correct.
Acetaldehyde is a chemical compound with formula, CH3CHO. And in the case of aldehyde, one or more monomer units of aldehyde are reacted together and there is a formation of aldehyde polymer. Hence, the option (D) is correct.
Note:
In the case of polymerization reaction, one or more monomer units react together to form a large unit of polymer with high molecular mass molecules. The polymerization reaction is mainly in two types and that is, chain reaction otherwise it is known as addition reaction and step reaction or condensation polymerization. And by polymerization process we can produce different types of plastic products.
