Question
Question: Which of these statements is true for the following molecule? ...
Which of these statements is true for the following molecule?
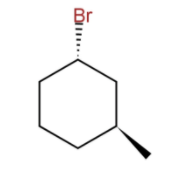
A) The bromine and methyl group are cis to one another.
B) The bromine and methyl group are trans to one another.
C) The bromine and methyl group are both axial.
D) The bromine and methyl group are both equatorial.
Solution
Hint: In wedge and dash chemical structures: solid triangular wedges represent groups or bonds coming out of the pages (towards us) while the dashed triangular shapes represent groups or bonds extending back behind the page.
Complete step by step answer:
-As we see in the above given structure of 1-Bromo-3-methylcyclohexane (C7H13Br):

The bond connecting the hexane ring to bromine is a dash type bond, which means that if we imagine a plane surface to be a hexane ring then this bond is extending backwards behind the plane surface and hence bromine is placed to the back of the surface.
The bond connecting hexane to the methyl group is a wedge type bond which means that if a plane surface is hexane then this bond is extending out of the plane in the front (towards us) and hence the methyl group is placed to the front of the plane.
Now, try and imagine this 3D image in your mind and start ruling out the false options.
Since now we know that Br is placed behind the plane and methyl is placed to the front of the plane, they both are in opposite directions. So, they cannot be at cis to each other.
Thus, option (A) is false.
Hence due to being in opposite planes or directions to each other we can say that they are at trans to each other.
So, option (B) is true.
Let's see the other 2 options also.
Br and methyl being in opposite planes cannot be either both axial or both equatorial to each other. If Br is axial then methyl would be equatorial and if Br is equatorial then methyl would be axial.
This rules out options (C) and (D).
So, the correct option is: (B) The bromine and methyl group are trans to one another.
Hint: The main mistake we make here is in imagining the 3D image of the compound. The question entirely depends on this so always make sure to imagine it right. Remember wedge bond means to the front of the plane and dash bond means to the behind of the plane.
