Question
Question: Which of these is the correct group reagent for group cations? A. \[M{{n}^{2+}}\text{, }C{{o}^{2+}...
Which of these is the correct group reagent for group cations?
A. Mn2+, Co2+,Zn2+, Ni2+dil.HCl
B. Mn2+, Co2+,Zn2+, Ni2+ NH4Cl+ NH4OH+H2S
C. Mn2+, Co2+,Zn2+, Ni2+ NH4Cl+ NH4OH
D. Mn2+, Co2+,Zn2+, Ni2+HCl + H2S
Solution
Hint: To solve this question we should know that Group reagent is a mixing of reagents that serves as a prime indication of a particular cation, characterized by the formation of a precipitate during a positive test/result and no precipitation in the event of a negative test/result.
Step by step answer:
We should know that all the cations present in these options are from D block. These are transitional elements. We should know that Zinc salts are colourless, Manganese salts are faint pink or colourless, and Nickel and cobalt salts may be brightly coloured, often blue-green. We should carefully observe the following diagram:
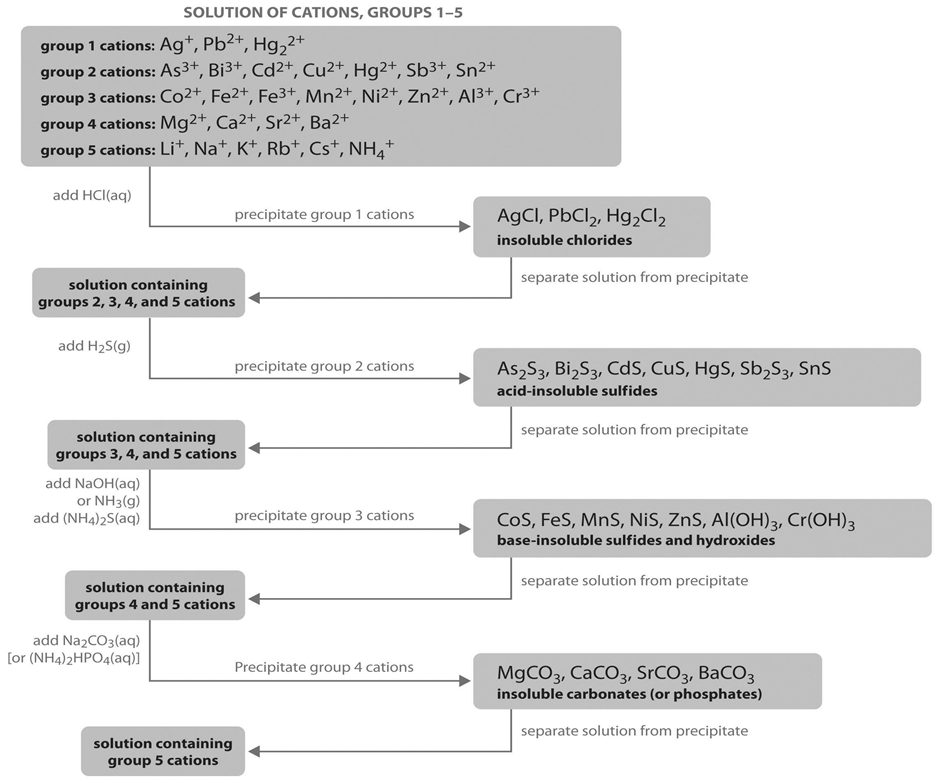
The precipitate, washed in water, reacts with extremely dilute hydrochloric acid. This precipitates nickel salts if any. The supernatant liquid is filtered and reacted with an excess of Sodium Hydroxide. This precipitates any Manganese salts. Hydrogen sulphide is passed through the supernatant liquid. If a white precipitate forms, Zinc is present. So, option B is correct.
Note: We should note that the composition of relatively complex mixtures of metal ions can be determined using qualitative analysis, a procedure for discovering the identity of metal ions present in the mixture. The procedure used to separate and identify more than 20 common metal cations from a single solution consists of selectively precipitating only a few kinds of metal ions at a time under given sets of conditions.
