Question
Question: Which of these compounds represents the major monobromination isomer formed in the following reactio...
Which of these compounds represents the major monobromination isomer formed in the following reaction?
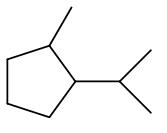
 major product
major product
(A) 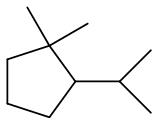
(B) 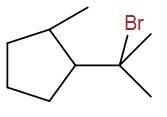
(C) 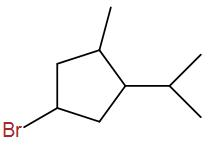
(D) 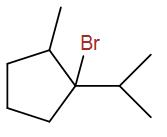
Solution
Hint : In the reaction, free radical mechanism is the path through which this reaction takes place. The bromine molecule forms reactive bromine radicals in the presence of UV rays. In a free radical mechanism, tertiary product is preferred.
Complete Step by step solution
The free radical mechanism is the mechanism in which the radical substitution reaction takes place. It results in the substitution of one or more atoms or groups present in the substrate by different atoms. In the free radical mechanism, there are three steps. The free radical mechanism starts with the chain initiation step. After the chain initiation step, there is a chain propagation step. It ends with the chain termination step. The reaction of the organic compound with bromine with ultraviolet light proceeds through the free radical mechanism. UV light splits the bromine molecule into two bromine radicals. These bromine radicals are reactive radicals.
There are three types of radicals formed.
They are primary radical, secondary radical and tertiary radical. The primary radical is the radical attached to the primary carbon, the secondary radical is the radical attached to the secondary carbon and the tertiary radical is the radical attached to the tertiary carbon. Of all the three radicals, tertiary radical is the most stable.
In the reaction, there are all types of carbon and hence all types of radicals can be formed. But the more stable is tertiary radical and hence the reaction that takes place is as follows,
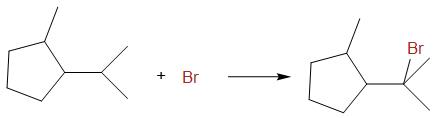
The correct answer is option B.
Note
Monobromination is a substitute of one bromine radical in organic compounds. Instead of bromine, chlorine can also be used.
