Question
Question: Which of the statements given below is incorrect? (A) ONF is isoelectronic with \({O_2}{N^ - }\) ...
Which of the statements given below is incorrect?
(A) ONF is isoelectronic with O2N−
(B) O2F is an oxide of fluorine
(C) Cl2O7 is an anhydride of perchloric acid
(D) O3 molecule is bent
Solution
Isoelectronic species are the species that have the same number of electrons in their structure. Oxides are the compounds in which oxygen atoms are in (-2) oxidation state.
Complete step by step solution:
We will check all the statements one by one in order to find whether they are true or not.
A) - Isoelectronic species are the species that have the same number of electrons in their structure.
- We are given two compounds. We know that each atom contains electrons equal to its atomic number.
- In ONF, O, N and F have atomic numbers of 8, 7 and 9 respectively. So, the total number of electrons in this compound will be 8+7+9=24 electrons.
- O2N− has an overall charge of (-1) as well. So, the total number of electrons will be 2(8)+7+1=24 electrons.
Thus, we can say that both the compounds are isoelectronic.
B) Oxides are the compounds in which oxygen atoms are in (-2) oxidation state. Here, oxygen atoms are in (+0.5) oxidation state. Fluorine is the more electronegative element among these two. So, O2F is a fluoride of oxygen. So, the statement given in option is incorrect.
C) Perchloric acid has a molecular formula HClO4. It molecular structure is
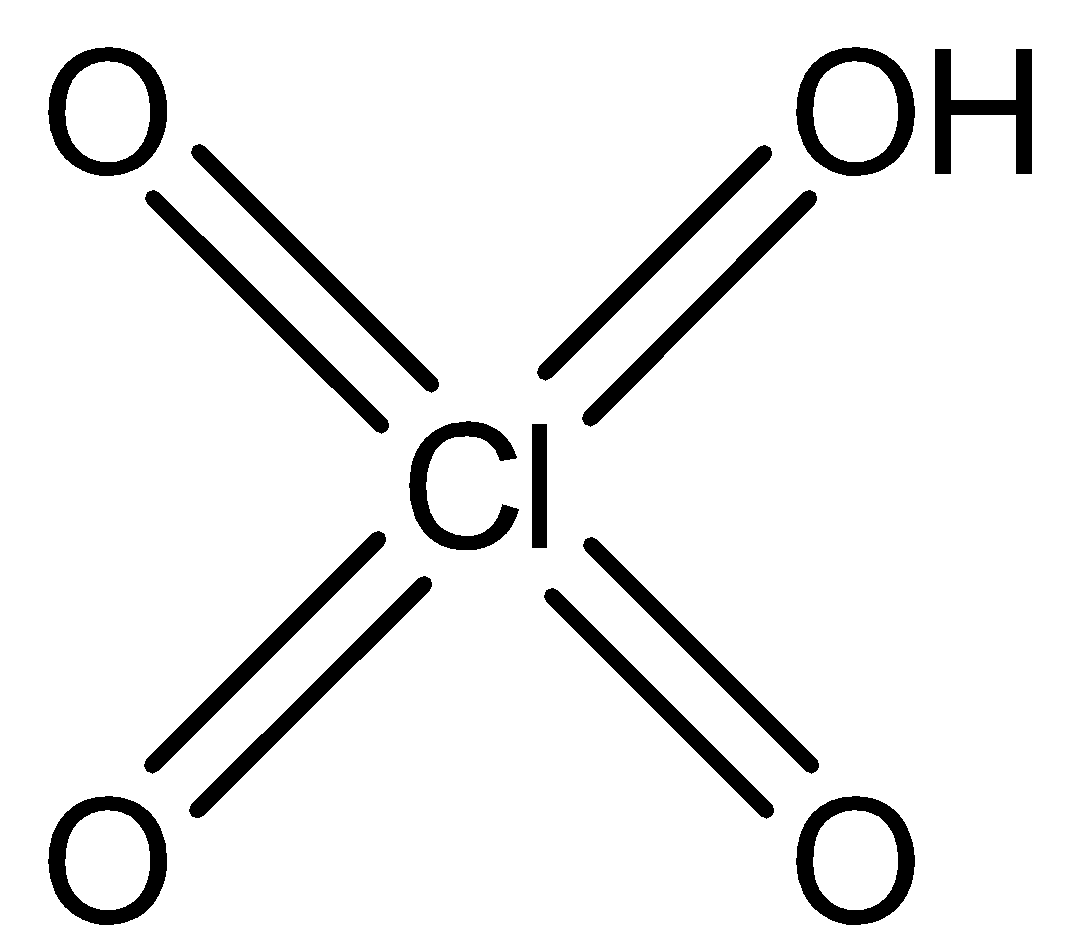
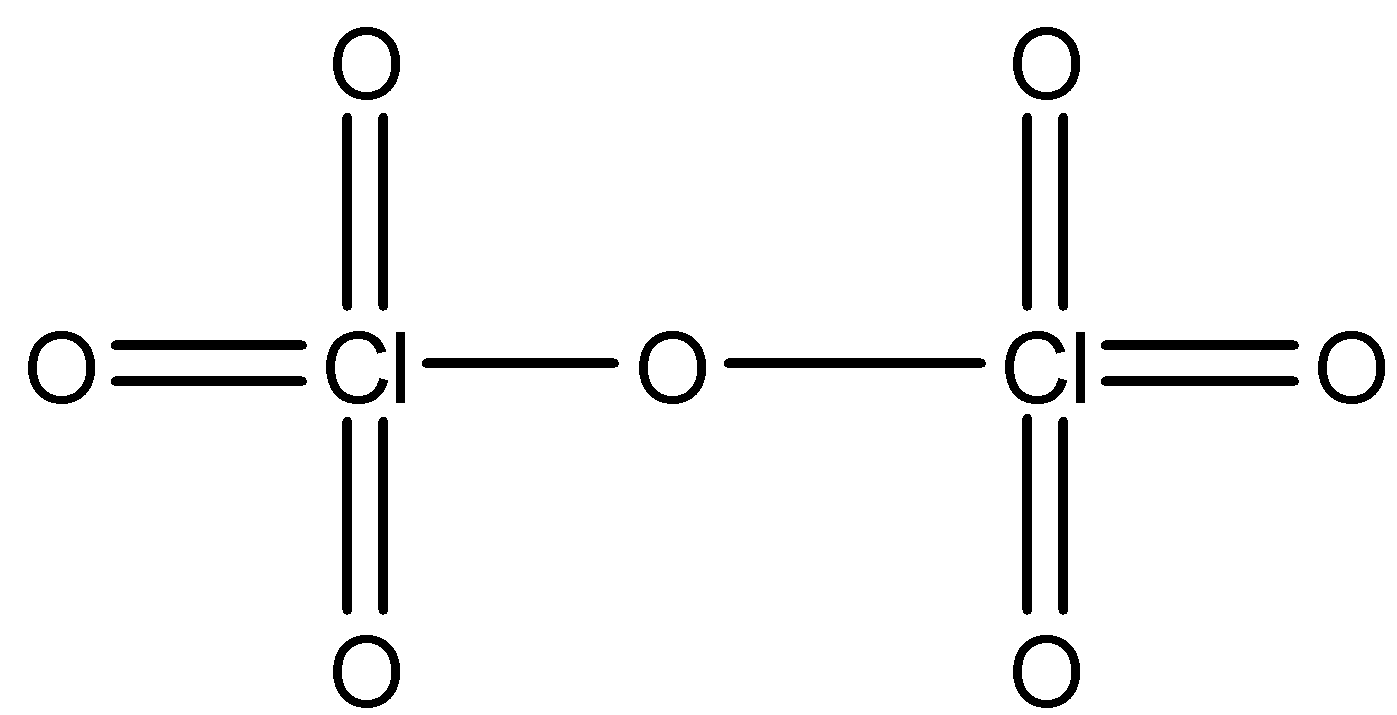
- Cl2O7 is actually an anhydride of perchloric acid because it has Cl-O-Cl type of linkage. Its structure is shown as below.
D) The ozone molecule is bent in shape because of the presence of lone pairs on the oxygen atoms. Its structure can be shown below.
Thus, we can conclude that option (B) is incorrect.
Note: Do not get confused between the names of oxyacids of chlorine. Some of them are as below:
Hypochlorous acid : HOCl
Chlorous acid: HClO2
Chloric acid: HClO3
Perchloric acid: HClO4
