Question
Question: Which of the molecules is trigonal bipyramidal? A. \(B{{F}_{3}}\) B. \(C{{H}_{4}}\) C. \(PC{...
Which of the molecules is trigonal bipyramidal?
A. BF3
B. CH4
C. PCl5
D. SF6
Solution
The number of bonds, lone pair of electrons, and the hybridization of the central atom explain the shape of the molecules. If there is a presence of a lone pair of electrons on the central atom the shape of the molecule is going to change.
Complete step by step answer:
- In the question, it is asked which molecules have the shape of trigonal bipyramidal among the given options.
- First, we should know the number of lone pairs on the central atom, the number of bonds, and the hybridization of the given molecules to find the shape of the given molecules.
- Coming to option A, BF3. The boron atom does not contain any lone pair of electrons on the central atom, the hybridization of the molecule is sp2 and the number of bonds is three then the structure of BF3 is trigonal planar and it is as follows.
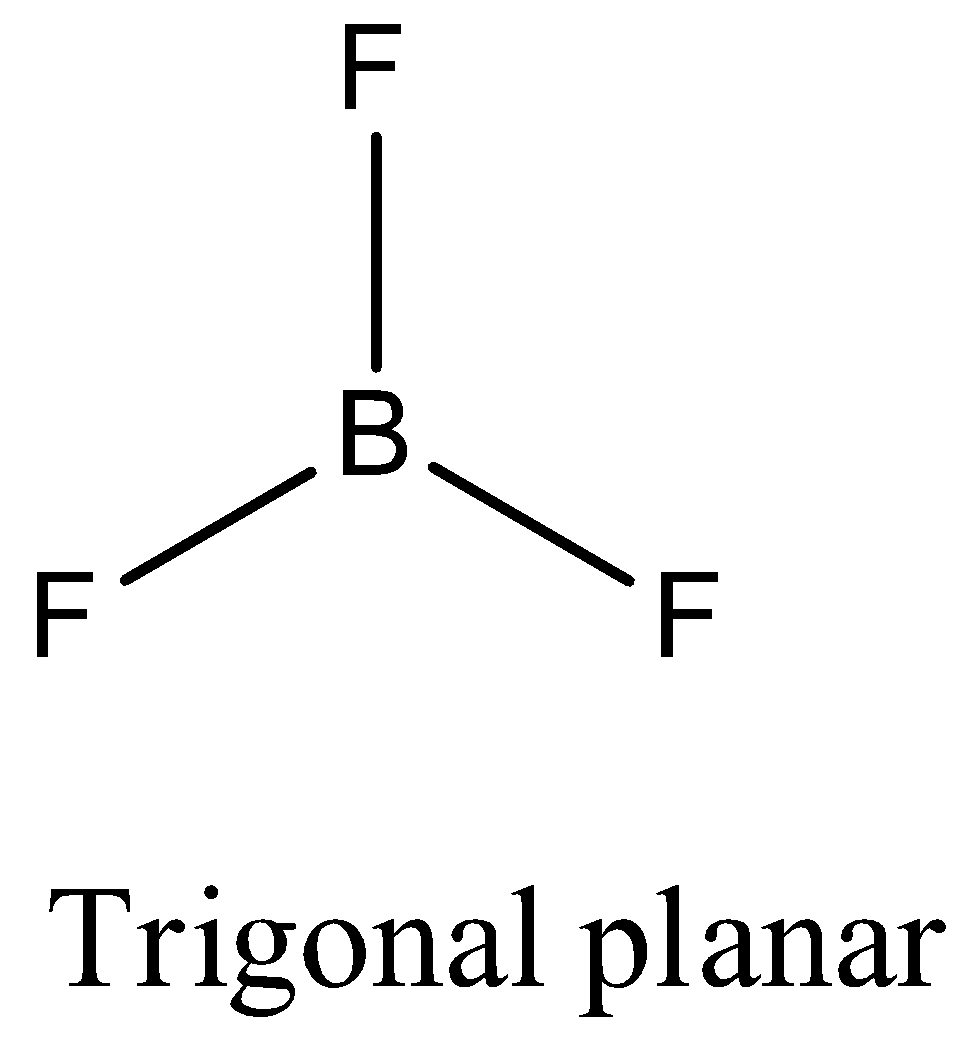
- Coming to option B, CH4 . The carbon atom does not contain any lone pair of electrons on the central atom, the hybridization of the molecule is sp3 and the numbers of bonds are four then the structure of CH4 is tetrahedral and it is as follows.
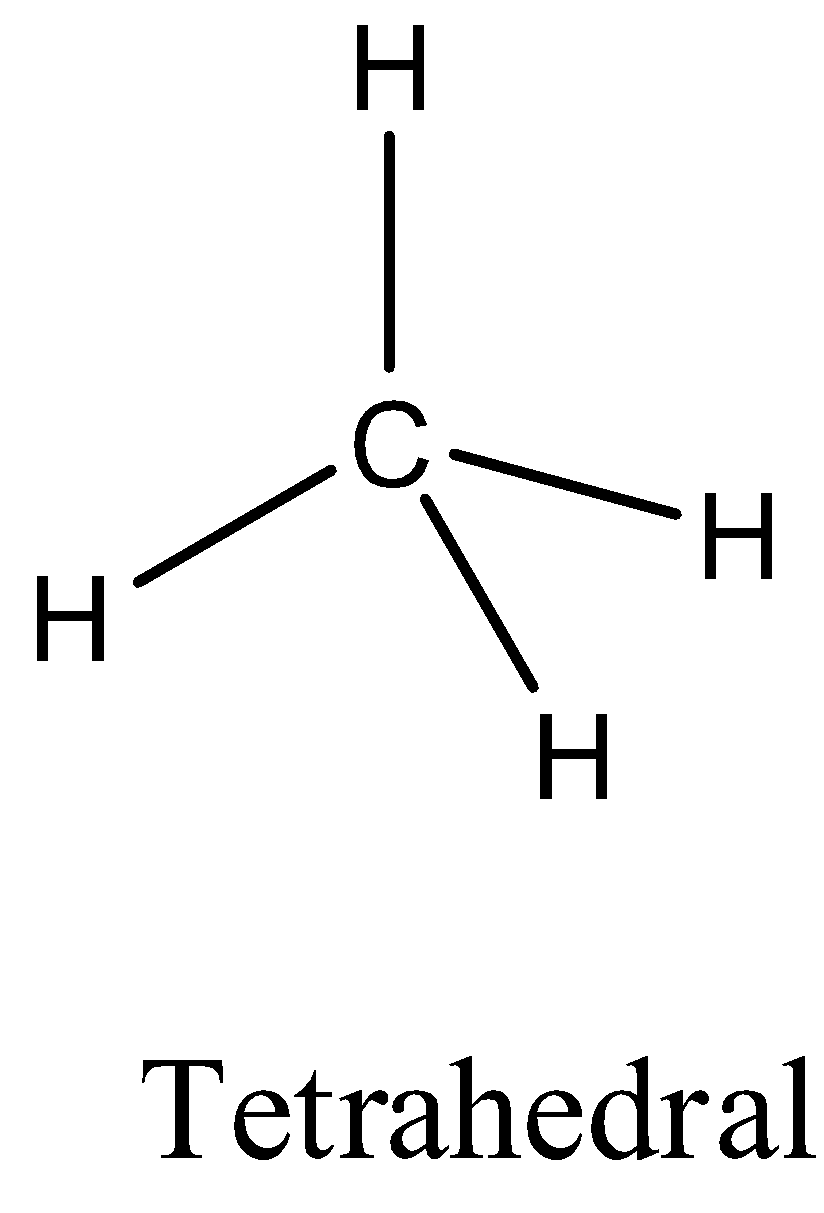
- Coming to the option C, PCl5 . The phosphorus atom does not contain any lone pair of electrons on the central atom, the hybridization of the molecule is sp3dand the numbers of bonds are five then the structure of is trigonal bipyramidal and it is as follows.
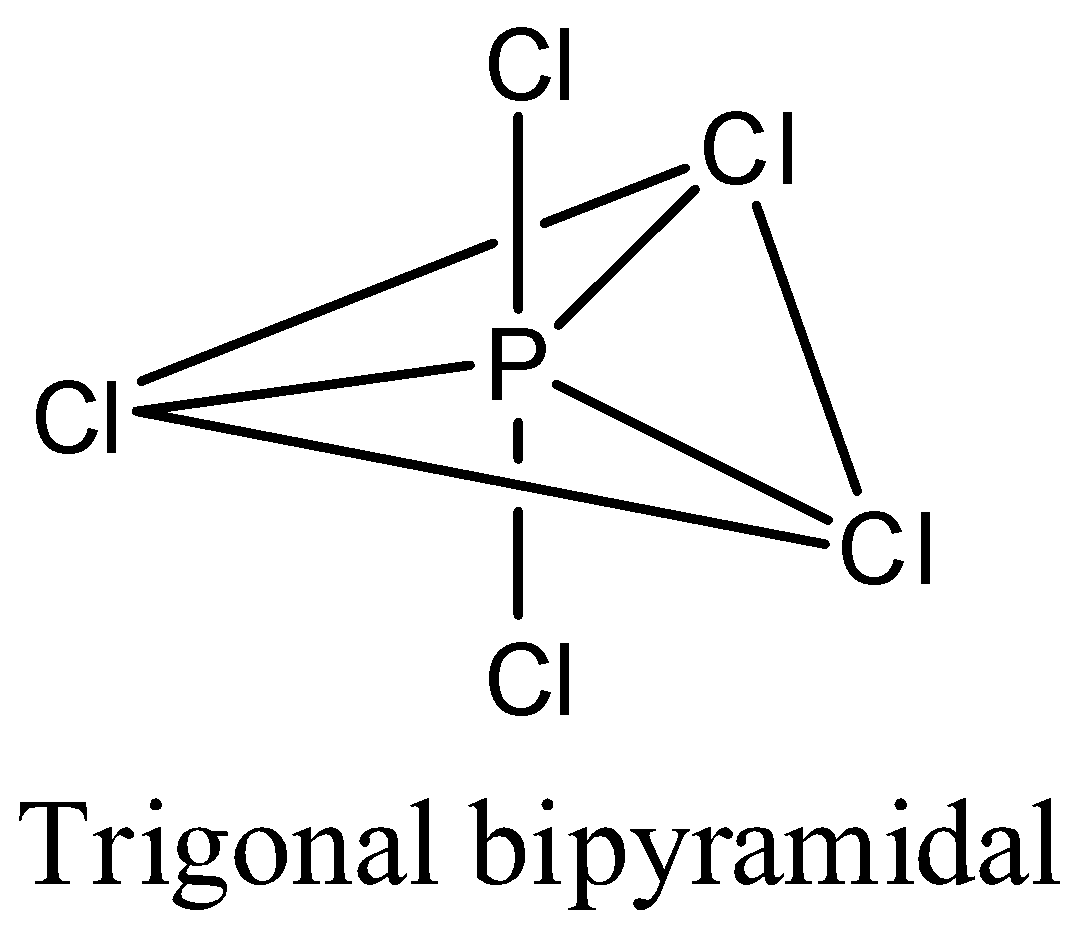
- Coming to the option D, SF6. The sulfur atom does not contain any lone pair of electrons on the central atom, the hybridization of the molecule is sp3d2 and the numbers of bonds are six then the structure of is octahedral and it is as follows.
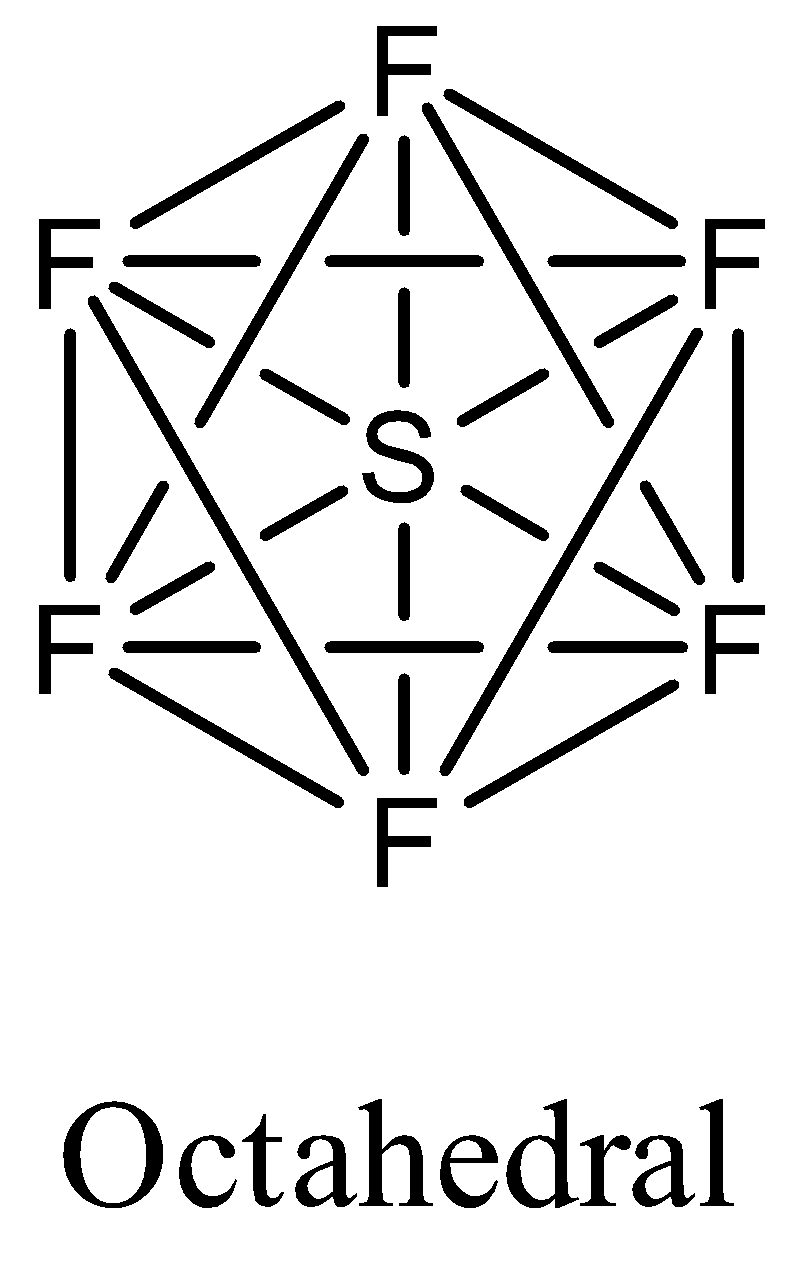
- Therefore the molecule which has trigonal bipyramidal structure among the given options is PCl5. So, the correct answer is “Option C”.
Note: If a lone pair of electrons are present on the central atom in the molecule then the shape of the molecule is going to change because of the repulsions between lone pair and bond pair electrons in the molecule.
