Question
Question: Which of the molecules is not tetrahedral? \(A.\) \(C{F_4}\) \(B.\) \(S{F_4}\) \(C.\) \(C{H_...
Which of the molecules is not tetrahedral?
A. CF4
B. SF4
C. CH4
D. SiF4
Solution
Four electrons pairs are distributed in a tetrahedral shape. If these are all bond pairs the molecular geometry is tetrahedral. If there is one lone pair of electrons and four bond pairs then the geometry is trigonal bipyramidal.
Complete step-by-step answer:
In the given question we have given four molecules namely CF4 , SF4 , CH4 and SiF4 . We have to find which one is not tetrahedral let check them one by one.
CF4 Molecule has four electron pairs all pairs are bond pairs so according to the VSEPR theory the shape of the molecule is tetrahedral.
In SF4 sulfur tetrafluoride has 5 region of electron density around the central atom (sulfur). In which four bonds and one lone pair, According to the VSEPR theory these are arranged in a trigonal bipyramidal shape.
In CH4 molecule has four electron pairs all the pairs are bond pair so, According to VSEPR theory these are arranged in a tetrahedral shape.
In the last molecule SiF4 have four electron pairs all the pairs are bond pair so, According to VSEPR theory these are arranged in a tetrahedral shape
Thus the correct answer is SF4 as its shape is trigonal bipyramidal.
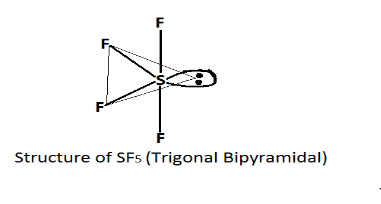
Note: Valence Shell Electron Pair Repulsion Theory (VSEPR) .This is a very useful theory to predict the geometry or shape of a number of polyatomic molecules or ions on a non-transition element. This theory says that shapes of a species depend on the number of and nature of electron pairs surrounding the central atom of a species.
Table summarizes the relationship between the number of electron pairs that are bond pairs and lone pairs and the shape or geometry.
| Total number of electron pairs | Shape |
|---|---|
| 2 | Linear |
| 3 | Trigonal planar |
| 4 | Tetrahedral |
| 5 | Trigonal bipyramidal |
| 6 | Octahedral |
| 7 | Pentagonal bipyramidal |
