Question
Question: Which of the molecule/species has \[{d^3}s\] hybridization? (A) \(Cr{O_2}C{l_2}\) (B) \(PC{l_4}^...
Which of the molecule/species has d3s hybridization?
(A) CrO2Cl2
(B) PCl4+
(C) NH4+
(D) ClO3−
Solution
The term ‘hybridization’ referred to as the process of mixing of atomic orbitals which have minute differences in their energies and these energies are redistributed as to form new atomic orbitals of equivalent energies and have similar shape. The concept of hybridization came into existence because valence bond theory failed to predict the molecular structure of some molecule.
Complete answer:
The chemical name of the compound with structure CrO2Cl2 is chromyl chloride. It is a coordinate compound with tetrahedral geometry. It is an inorganic compound.
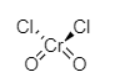
The chromium atom in CrO2Cl2 undergoes d3s hybridisation. The oxidation state of chromium in chromyl chloride is +6.
The electronic configuration of chromium is given as [Ar]3d54s1
In d3s hybridization of chromium, the atomic orbitals involved are 4s, 3dxy, 3dyz and 3dxz.
**So, the correct answer is Option (A) CrO2Cl2
Note: **
Students might get confused about which hybridisation is taking place in chromyl chloride. As the complex has tetrahedral geometry, students might think that the central chromium atom undergoes sp3 hybridisation. That is not true. The central chromium atom undergoes d3s hybridization.
