Question
Question: Which of the following would have a permanent dipole moment? A. \(BF_{3} \) B. \(SiF_{4} \) C....
Which of the following would have a permanent dipole moment?
A. BF3
B. SiF4
C. SF4
D. XeF4
Solution
Hint : To solve the question we are going to draw the electron dot structure of the compounds and use valence shell electron pair repulsion theory to find the compound having maximum dipole moment.
Complete step by step answer:-
Electric Dipole moment is an arrangement of two equal and opposite charges placed at a distance from each other and the product of any charge and the distance between them gives electric dipole.
The VSEPR theory says that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence shell of that atom. Thus we can predict the shapes of given compounds and find the net dipole moment. Here are the structure of given compounds:-
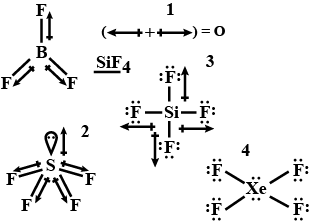
- In BF3, the dipole moments cancel each other
- SiF4 has a bent structure due to sp3d hybridization resulting in a net dipole moment.
- In SF4 there is no net permanent dipole due to cancellation of dipole moments.
- XeF4 is non polar due to planar structure.
So from the above observations it's clear that SF4 has a lone pair in its outer shell. Out of six electrons of s subshell, four electrons make a bond with F and two electrons remain as lone pairs.
**Hence option B is correct.
Note : **
The student may make a mistake in finding the correct direction of the dipole moment and the resultant of two dipole moments by vector laws.
