Question
Question: Which of the following will show the least dipole character? A. Water B. Ethanol C. Ethane D...
Which of the following will show the least dipole character?
A. Water
B. Ethanol
C. Ethane
D. Ether
Solution
Dipole moment of any molecule is the product of the charge and the distance between the centers of negative and positive charges. It is denoted by,μ, and has a unit called Debye. Dipole moment is a vector quantity and the sum of individual dipole moments is calculated in terms of polyatomic molecules. Dipole moment results in the polarity of a molecule.
Complete answer:
Dipole moment of any molecule is the measure of the distance between positive and negative charge into the net magnitude of the charge on the molecule. The shapes of molecules affect the resultant dipole. The bent shape molecules have high resultant dipoles as compared to the linear shaped molecules. The dipole is affected by the electronegativity of the attached atoms. This in turn affects the shape of molecules. Also the presence of a lone pair of electrons affects the resultant dipole and has a net dipole.
Water, ethanol, and ethers have bent shape due to the presence of lone pairs of electrons on oxygen atoms, which creates a net dipole of more than zero, μ>0, so these molecules will have a dipole character.
Ethane as compared to these molecules have a linear and symmetrical structure that reduces its dipole character.
Therefore, ethane molecules have the least dipole character among the molecules.
So, option C is correct.
Note:
The shapes of molecules affect the dipole as dipole depends on the distances. The shapes of the given
molecules are
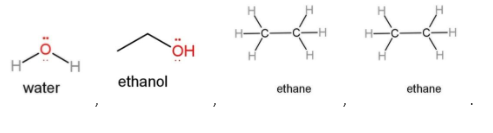
Which clearly shows that ethane will have a less dipole character as compared to the other molecules. Also, other molecules are polar that increases that dipole character.
