Question
Question: Which of the following will react with \(NaOI\)? A.
B.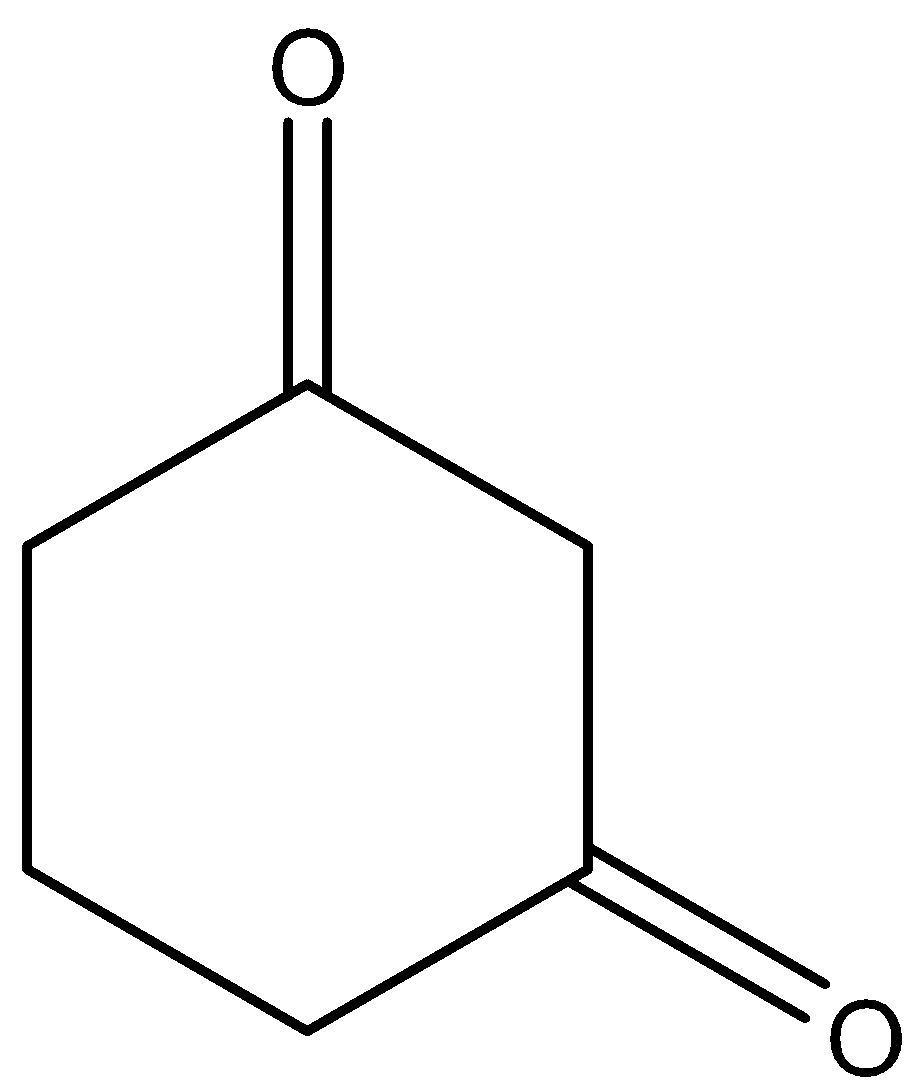
C.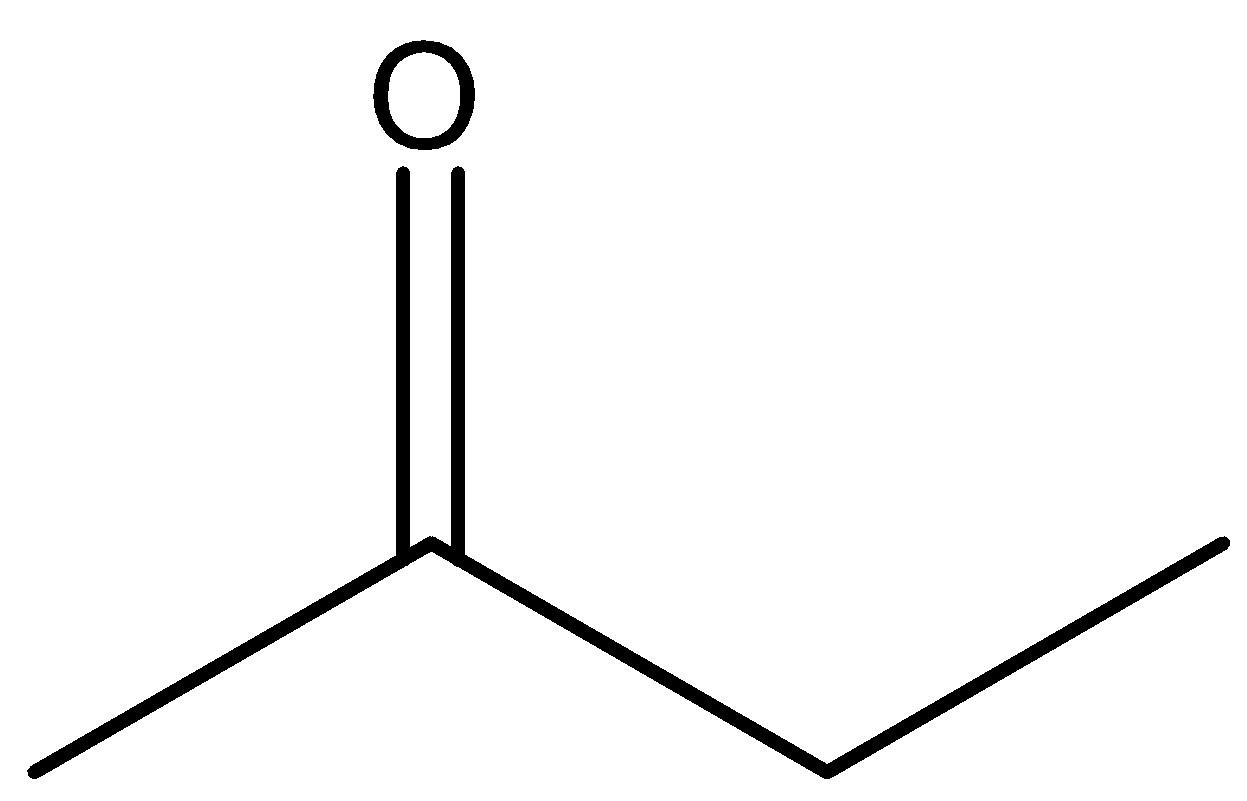
D.All
Solution
NaOI is known as sodium hypo iodide. It is formed by the reaction of I2 and NaOH used in the iodoform reaction. The alcohols, aldehydes and ketones containing CH3COgroup react with NaOI to form yellow precipitate of CHI3 which is called as iodoform. This reaction is used as a test for differentiating between different alcohols, aldehydes and ketones.
Complete answer:
If any molecule reacts with NaOI it should have CH3CO i.e.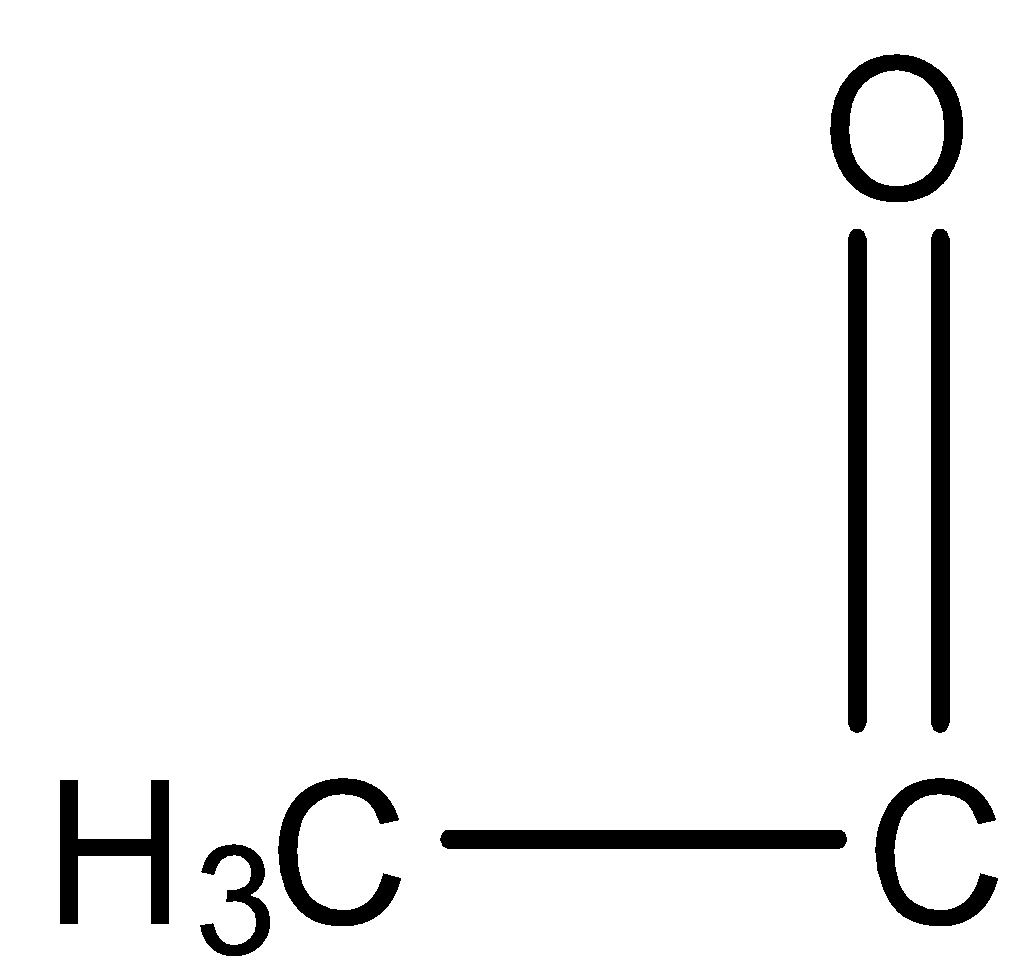 group and at the end of the reaction it will give CHI3 as a yellow precipitate. NaOI takes part in a reaction as NaOH and I2.
group and at the end of the reaction it will give CHI3 as a yellow precipitate. NaOI takes part in a reaction as NaOH and I2.
First let us look at basic reaction whereNaOI will react with 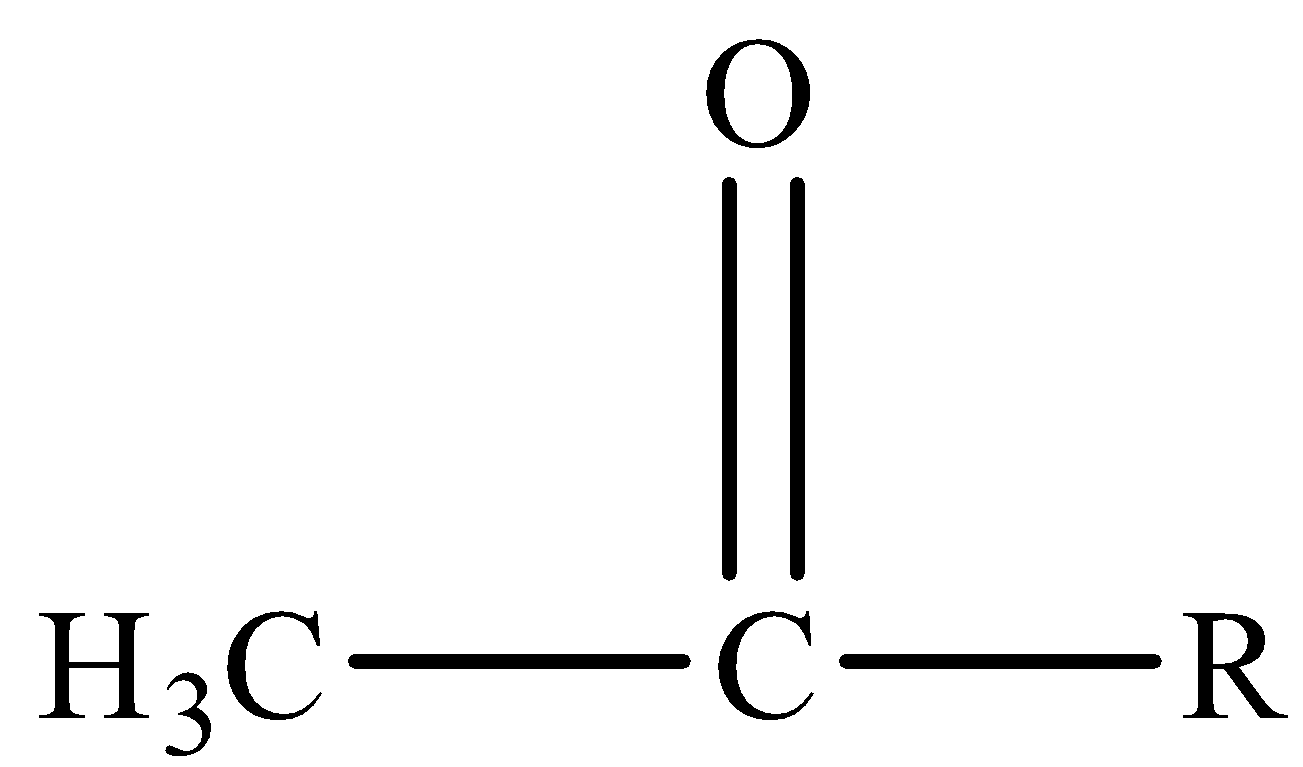 ( where R is any aromatic or aliphatic group)
( where R is any aromatic or aliphatic group)
The mechanism is as follows:
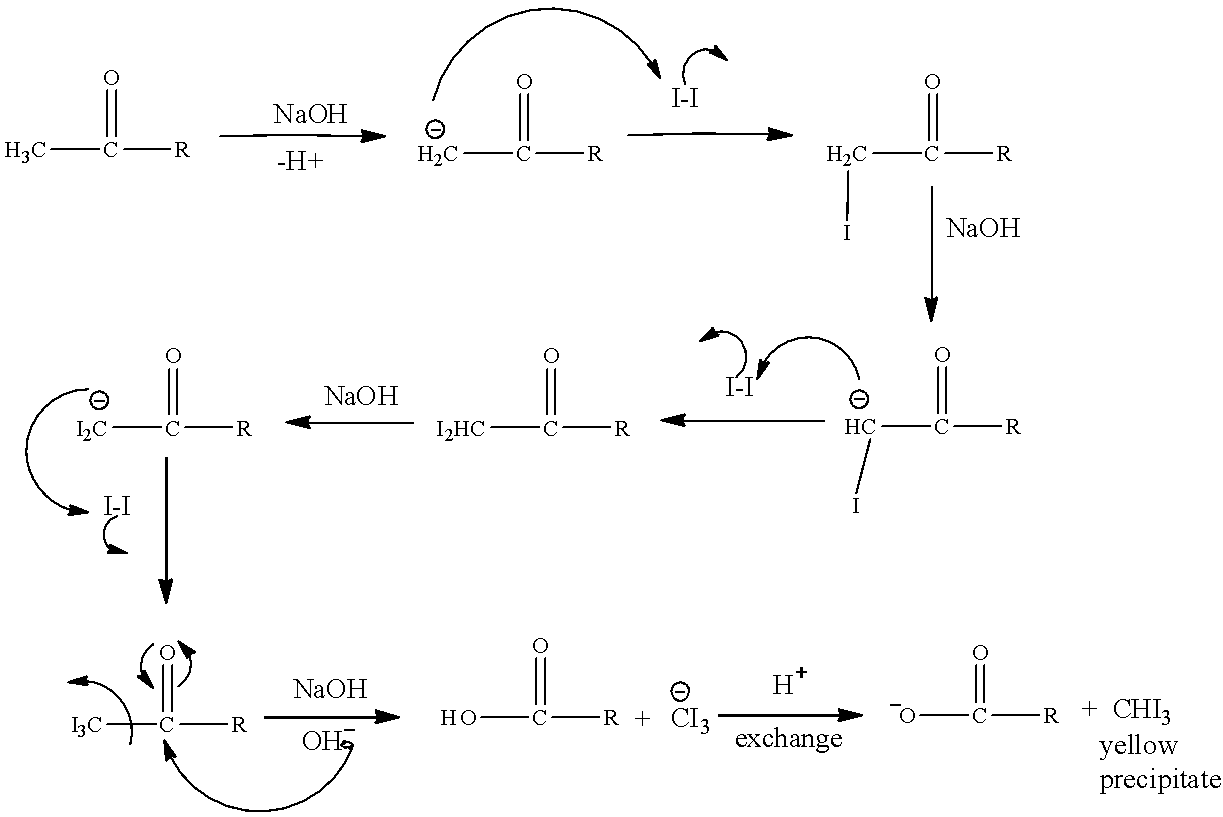
Now we can clearly see in the above mechanism how the CH3CO group containing molecules is undergoing reaction and in the end CHI3 is produced.
So among our options we say that 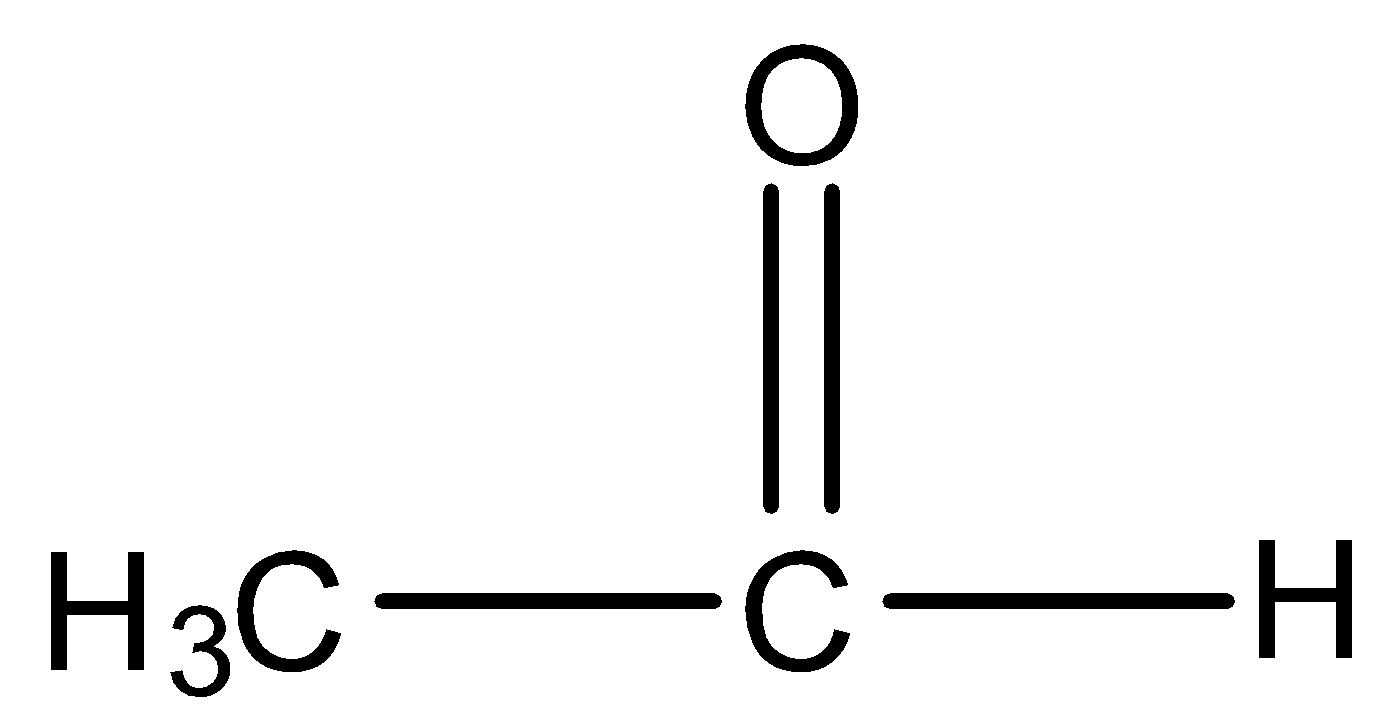 and
and 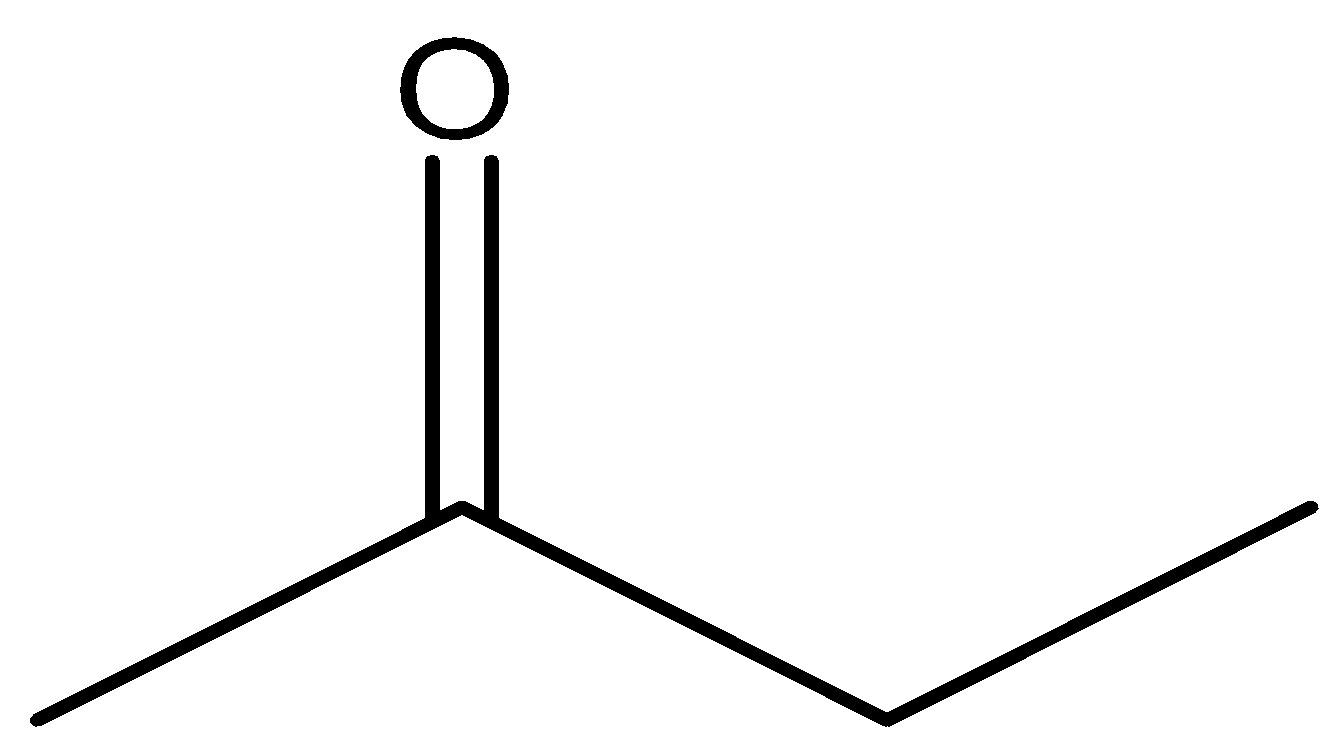 will react with NaOI to give the product as both the molecules contain CH3CO group in their structure. Now let us look at the aromatic compound.
will react with NaOI to give the product as both the molecules contain CH3CO group in their structure. Now let us look at the aromatic compound.
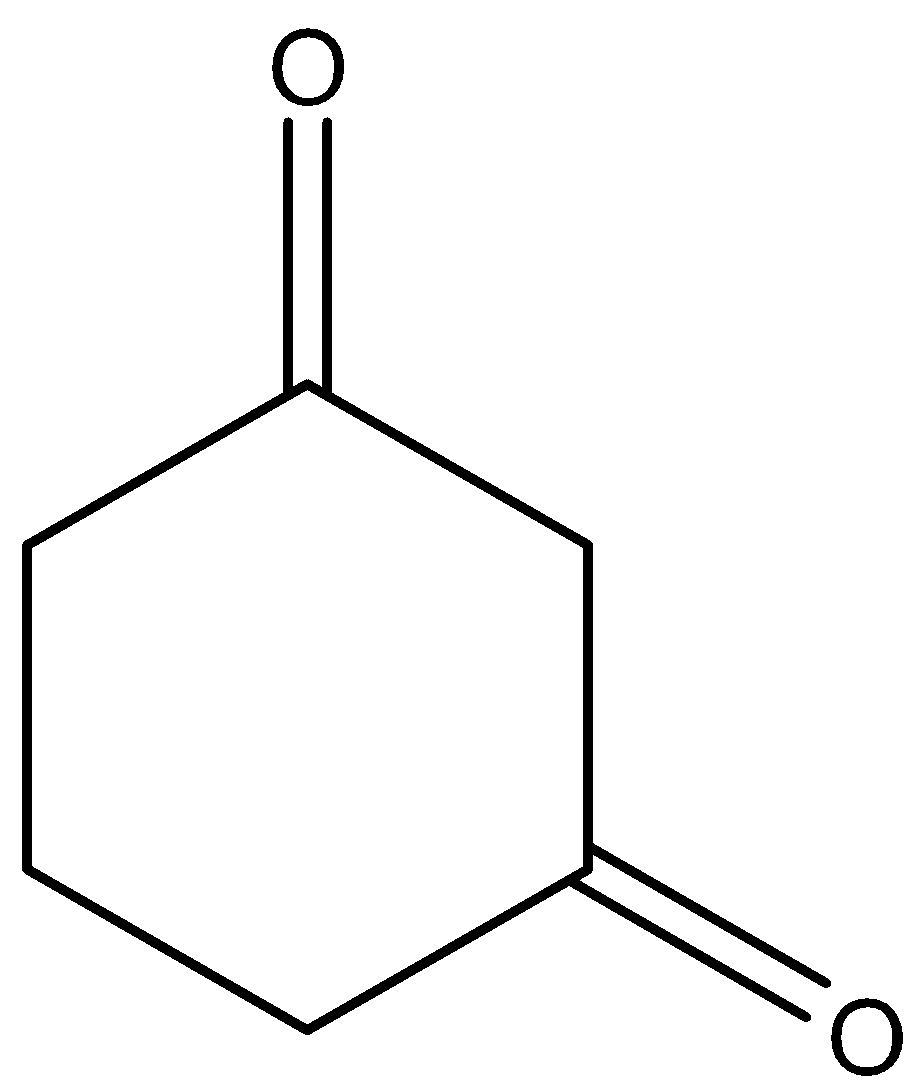 Here this molecule does not have any CH3CO group in its structure so for knowing whether it will react with NaOI or not we will have to look into its mechanism, which is as follows:
Here this molecule does not have any CH3CO group in its structure so for knowing whether it will react with NaOI or not we will have to look into its mechanism, which is as follows:
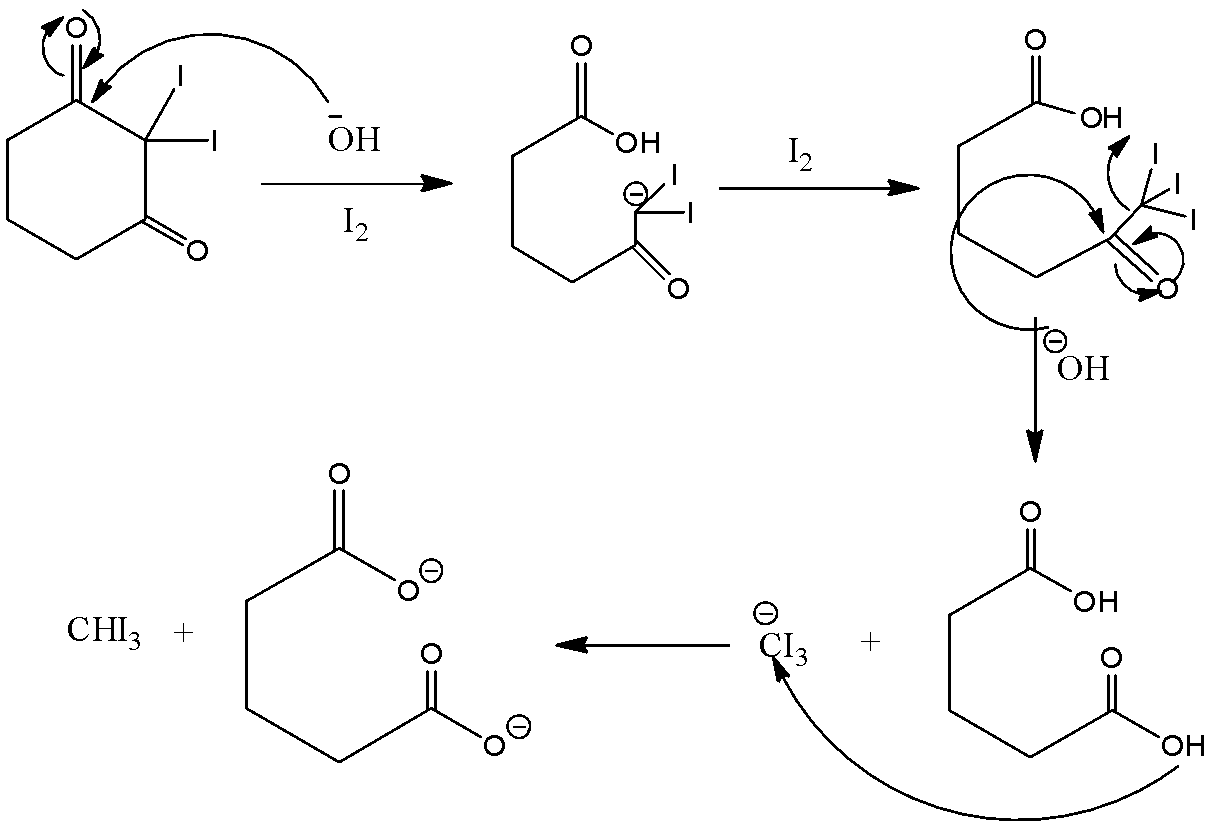
So in the above mechanism base will extract and then I will get attached to it twice, third time there is no Hleft so −OH will then act as nucleophile
We can see that the compound does not have any CH3CO group but it still reacts with NaOI to give CHI3
Therefore all the above molecules will react with NaOI.
Hence the correct option is D. All
Note:
Only those molecules will react with NaOI which haveCH3CO group in them and yield CHI3 as a precipitate. Even if the molecule does not haveCH3CO, in some cases it may react with NaOI and give the yellow precipitate as in the above case.
